A1501
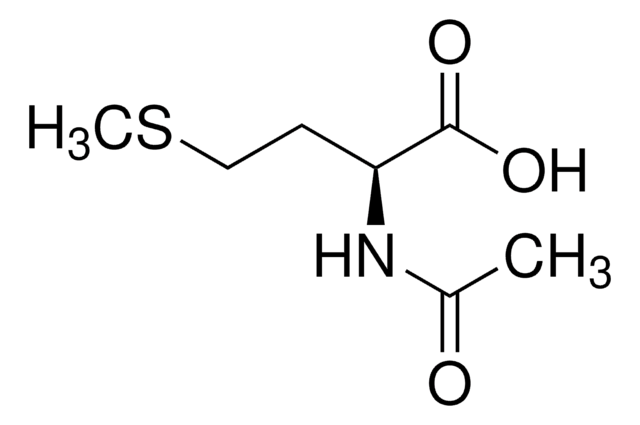
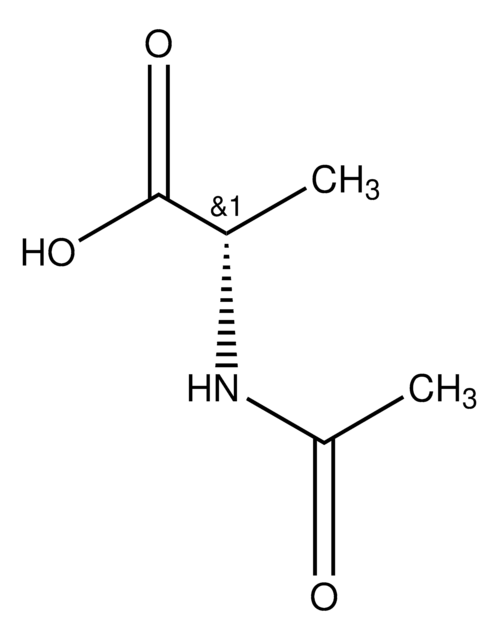
N-Acetyl-D-methionine
~99%, suitable for ligand binding assays
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C7H13NO3S
Numero CAS:
Peso molecolare:
191.25
Beilstein:
1725553
Numero CE:
Numero MDL:
Codice UNSPSC:
12352209
eCl@ss:
32160406
ID PubChem:
NACRES:
NA.26
Prodotti consigliati
Nome del prodotto
N-Acetyl-D-methionine, ~99%
Saggio
~99%
Stato
powder or crystals
tecniche
ligand binding assay: suitable
Colore
white
Punto di fusione
102.3-103.6 °C
Temperatura di conservazione
−20°C
Stringa SMILE
CSCC[C@@H](NC(C)=O)C(O)=O
InChI
1S/C7H13NO3S/c1-5(9)8-6(7(10)11)3-4-12-2/h6H,3-4H2,1-2H3,(H,8,9)(H,10,11)/t6-/m1/s1
XUYPXLNMDZIRQH-ZCFIWIBFSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
N-Acetyl-D-methionine may be used as a substrate to identify, differentiate and characterized N-acylamino acid racemase(s) and N-acyl-D-amino acid amidohydrolase(s).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Wen-Ching Wang et al.
Journal of molecular biology, 342(1), 155-169 (2004-08-18)
N-acylamino acid racemase (NAAAR) catalyzes the racemization of N-acylamino acids and can be used in concert with an aminoacylase to produce enantiopure alpha-amino acids, a process that has potential industrial applications. Here we have cloned and characterized an NAAAR homologue
Pei-Hsun Lin et al.
European journal of biochemistry, 269(19), 4868-4878 (2002-10-02)
An N-acyl-d-amino acid amidohydrolase (N-D-AAase) was identified in cell extracts of a strain, Iso1, isolated from an environment containing N-acetyl-d-methionine. The bacterium was classified as Variovorax paradoxus by phylogenetic analysis. The gene was cloned and sequenced. The gene consisted of
S Pittelkow et al.
Protein expression and purification, 12(2), 269-276 (1998-03-31)
Aminoacylase I (EC 3.5.1.14) is one of the most abundant enzymes in the cortical region of mammalian kidney. Both the porcine and the human enzyme were overexpressed using baculovirus expression vector systems and purified by hydrophobic interaction chromatography and anion-exchange
T Odajima et al.
Cell biochemistry and function, 16(2), 139-147 (1998-06-24)
Urate oxidase from Candida utilis, an enzyme containing an essential thiol, was examined for its sensitivity to lactoperoxidase, an oxidant present in breast milk. Upon exposure to a system composed of lactoperoxidase, hydrogen peroxide and bromide at moderately alkaline pH
M J Wick et al.
Biochemical pharmacology, 37(7), 1225-1231 (1988-04-01)
Both N-hydroxy-2-acetamidofluorene (N-OH-AAF) and the heterocyclic analogue, 2-(N-hydroxyacetamido)carbazole (N-OH-AAC), were shown to be mechanism-based irreversible inhibitors (suicide inhibitors) of partially purified rat hepatic N-acetyltransferase (NAT) activity. Although N-OH-AAC exhibited an apparent first-order inactivation rate constant (ki) that was 7-fold lower
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.