208221
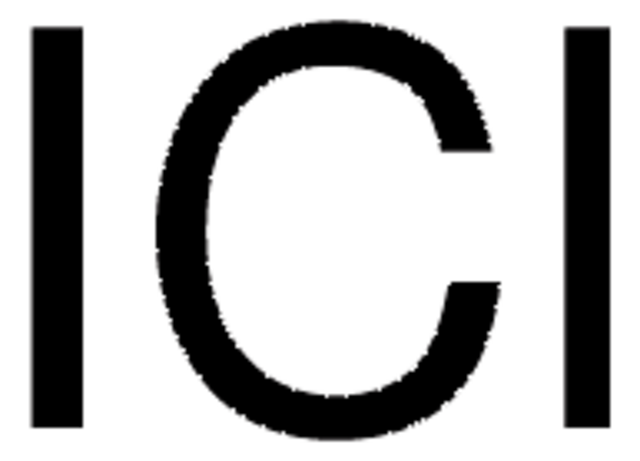
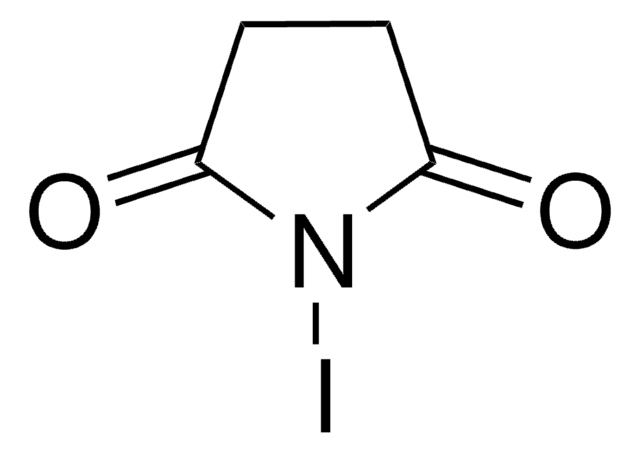
Iodine monochloride
reagent grade, ≥95%
Sinonimo/i:
Chloroiodide
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
ICl
Numero CAS:
Peso molecolare:
162.36
Numero CE:
Numero MDL:
Codice UNSPSC:
12352300
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Grado
reagent grade
Livello qualitativo
Saggio
≥95%
Stato
solid or liquid
P. ebollizione
97.4 °C (lit.)
Densità
3.24 g/mL at 25 °C (lit.)
Temperatura di conservazione
2-8°C
Stringa SMILE
ClI
InChI
1S/ClI/c1-2
QZRGKCOWNLSUDK-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Iodine monochloride is an interhalogen compound. It forms various complexes with ethyl, isopropyl and t-butylbenzenes. Electrically conducting solution of ICl is obtained on dissolution of ICl in polar solvents. ICl is a green oxidizing agent and participates in the following transformations:
- aldose hemiacetals to the corresponding aldose lactones
- diarylmethanols to the corresponding diarylmethanones
- arylalkylmethanols to the corresponding arylalkylmethanones
- dialkylmethanols to the corresponding dialkylmethanones
Applicazioni
Iodine monochloride (ICl) may be employed as a reagent in the following processes:
- Halogenation of methoxy and dimethoxybenzenes
- Synthesis of flavones.
- Preparation of 1-naphthaldehydes.
Avvertenze
Danger
Indicazioni di pericolo
Classi di pericolo
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
8B - Non-combustible corrosive hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Iodine Monochloride (ICl) as a Highly Efficient, Green Oxidant for the Oxidation of Alcohols to Corresponding Carbonyl Compounds
Wei, Peng, et al.
Synthetic Communications, 45.12, 1457-1470 (2015)
Synthesis of 1-naphthaldehydes via the cascade reactions of 1-phenylpent-4-yn-2-ols promoted by iodine monochloride
Li B, et al.
Tetrahedron Letters, 57.17, 1843-1846 (2016)
A mild and convenient procedure for conversion of alkenes into alkyl iodides via reaction of iodine monochloride with organoboranes.
Kabalka GW and Gooch III EE.
The Journal of Organic Chemistry, 45(18), 3578-3580 (1980)
Cation radicals as intermediates in aromatic halogenation with iodine monochloride: solvent and salt effects on the competition between chlorination and iodination.
Hubig SM, et al.
The Journal of Organic Chemistry, 59(21), 6233-6244 (1994)
Ultrasonic-assisted synthesis of flavones by oxidative cyclization of 2'-hydroxychalcones using iodine monochloride.
Lahyani A and Trabelsi M.
Ultrasonics Sonochemistry, 31, 626-630 (2016)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.