Y0000319
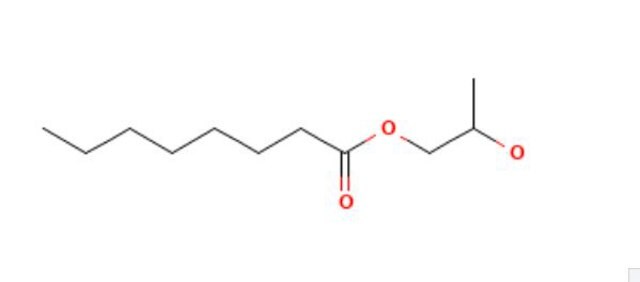
Propylene glycol monolaurate
European Pharmacopoeia (EP) Reference Standard
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C15H30O3
Numero CAS:
Peso molecolare:
258.40
Numero CE:
Codice UNSPSC:
41116107
NACRES:
NA.24
Prodotti consigliati
Grado
pharmaceutical primary standard
Famiglia di API
propylene glycol
Produttore/marchio commerciale
EDQM
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
2-8°C
InChI
1S/C15H30O3/c1-3-4-5-6-7-8-9-10-11-12-15(17)18-13-14(2)16/h14,16H,3-13H2,1-2H3
BHIZVZJETFVJMJ-UHFFFAOYSA-N
Descrizione generale
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Applicazioni
Propylene glycol monolaurate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Confezionamento
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Altre note
Sales restrictions may apply.
Prodotti correlati
N° Catalogo
Descrizione
Determinazione del prezzo
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Lot/Batch Number
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Rana Abu-Huwaij et al.
Drug development and industrial pharmacy, 33(4), 437-448 (2007-05-25)
The aim of this study was to develop a controlled release buccal mucoadhesive delivery system for systemic delivery of lidocaine hydrochloride as a model drug. In vitro release and buccal permeation as well as in vivo permeation of LDHCL patches
Sang-Chul Shin et al.
Archives of pharmacal research, 29(10), 928-933 (2006-11-24)
Percutaneous delivery of NSAIDs has advantages of avoiding hepatic first pass effect and delivering the drug for extended period of time at a sustained, concentrated level at the inflammation site that mainly acts at the joint and the related regions.
B R Jasti et al.
The journal of investigative dermatology. Symposium proceedings, 3(2), 128-130 (1998-09-12)
Excipients are often used in transdermal formulations to overcome the formidable barrier offered by the epidermis in order to achieve the target flux. In this study we describe the use of frequency-domain fluorescence spectroscopy to characterize the effect of two
Jian Meng et al.
Drug development and industrial pharmacy, 33(9), 927-931 (2007-09-25)
Self-microemulsifying drug delivery systems (SMEDDS) are useful to improve the bioavailability of poorly water-soluble drugs by increasing their apparent solubility through solubilization. However, very few studies, to date, have systematically examined the level of drug apparent solubility in o/w microemulsion
Archita Patel et al.
Current drug delivery, 12(6), 745-760 (2015-03-04)
The solid-self nanoemulsifying drug delivery system (S-SNEDDS) of Amiodarone hydrochloride (AH) was prepared and evaluated. AH exhibits poor aqueous solubility (0.3-0.5 mg/ml) and therefore variable oral bioavailability. Capmul MCM, Cremophor RH-40 and Propylene glycol were identified as oil, surfactant and
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.