220201
CHAPS
Molecular Biology Grade
Sinonimo/i:
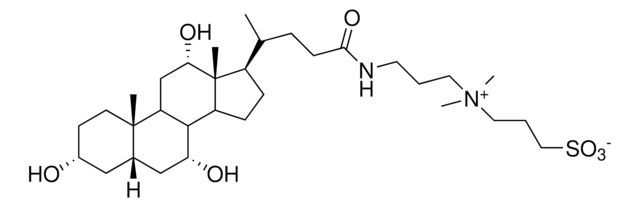
CHAPS, 3-[(3-Cholamidopropyl)dimethylammonio]-1-propanesulfonate
About This Item
Prodotti consigliati
Nome del prodotto
CHAPS, Molecular Biology Grade,
Grado
Molecular Biology
Livello qualitativo
Descrizione
zwitterionic
Saggio
≥98% (NMR)
Stato
crystalline powder
PM
micellar avg mol wt 6150
Produttore/marchio commerciale
Calbiochem®
Condizioni di stoccaggio
OK to freeze
desiccated (hygroscopic)
Numero d'aggregazione
10
Colore
white
CMC
6 - 10 mM
6 mM (20-25°C)
Punto di fusione
157 °C (315 °F)
Temp. transizione
cloud point >100 °C
Solubilità
water: 1.0 M
Attività estranea
DNase, RNase, and Protease, none detected
Condizioni di spedizione
ambient
Temperatura di conservazione
15-25°C
Stringa SMILE
C[C@H](CCC(=O)NCCC[N+](C)(C)CCCS([O-])(=O)=O)[C@H]1CC[C@H]2[C@@H]3[C@H](O)CC4C[C@H](O)CC[C@]4(C)[C@H]3C[C@H](O)[C@]12C
InChI
1S/C32H58N2O7S/c1-21(8-11-29(38)33-14-6-15-34(4,5)16-7-17-42(39,40)41)24-9-10-25-30-26(20-28(37)32(24,25)3)31(2)13-12-23(35)18-22(31)19-27(30)36/h21-28,30,35-37H,6-20H2,1-5H3,(H-,33,38,39,40,41)/t21-,22?,23-,24-,25+,26+,27-,28+,30+,31+,32-/m1/s1
UMCMPZBLKLEWAF-RFCNGIAKSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Useful for solubilizing membrane proteins and breaking protein-protein interactions. CHAPS′ small micellar molecular weight (6,150) and high critical micelle concentration (6-10 mM) allow it to be removed from samples by dialysis. It is also suitable for protein solubilization for isoelectric focusing and two-dimensional electrophoresis. CHAPS is commonly used for non-denaturing (without urea) IEF and has been shown to give excellent resolution of some subcellular preparations and plant proteins. Concentrations between 2-4% (w/v) are typically used in an IEF gel.
Attenzione
Ricostituzione
Altre note
Ofri, D., et al. 1992. J. Neurochem. 58, 628.
Ransom, R.W., et al. 1992. Biochem. Pharmacol. 43, 1823.
Simonds, W.F., et al. 1992. Proc. Natl. Acad. Sci. USA77, 4623.
Yannariello-Brown, J., and Weigel, P.H. 1992. Biochemistry31, 576.
Hjelmeland, L.M. 1980. Proc. Natl. Acad. Sci. USA77, 6368.
Simonds, W.F., et al. 1980. Proc. Natl. Acad. Sci. USA77, 4623.
Note legali
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![CHAPS (3-[(3-Cholamidopropyl)-dimethylammonio]-propane- sulfonate) for biochemistry](/deepweb/assets/sigmaaldrich/product/structures/265/550/452ffcaa-af89-4e31-99a1-0a0f29072b82/640/452ffcaa-af89-4e31-99a1-0a0f29072b82.png)