W263613
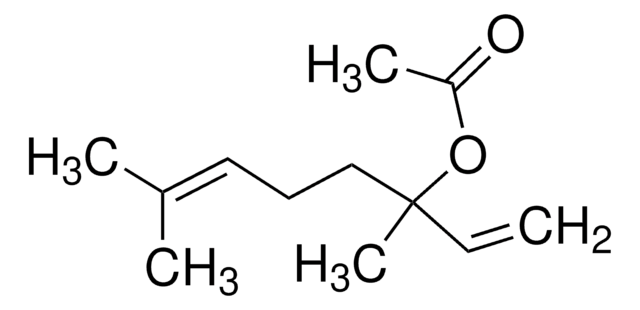
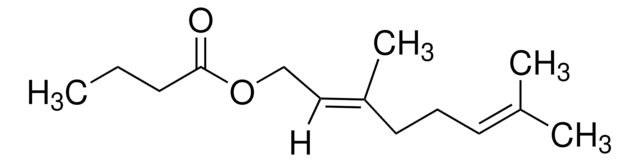
Linalyl acetate
natural, ≥96%, FG
Sinonimo/i:
3,7-Dimethyl-1,6-octadien-3-yl acetate, Bergamol
About This Item
Prodotti consigliati
Origine biologica
botanical
Grado
FG
Halal
Kosher
natural
Conformità normativa
EU Regulation 1334/2008 & 872/2012
FDA 21 CFR 117
Densità del vapore
6.8 (vs air)
Tensione di vapore
0.1 mmHg ( 20 °C)
Saggio
≥96%
Caratteristiche più verdi
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
Indice di rifrazione
n20/D 1.453 (lit.)
P. ebollizione
220 °C (lit.)
Densità
0.901 g/mL at 25 °C (lit.)
applicazioni
flavors and fragrances
Documentazione
see Safety & Documentation for available documents
Allergene alimentare
no known allergens
Categoria alternativa più verde
Organolettico
green; woody; floral; sweet
Stringa SMILE
C\C(C)=C\CCC(C)(OC(C)=O)C=C
InChI
1S/C12H20O2/c1-6-12(5,14-11(4)13)9-7-8-10(2)3/h6,8H,1,7,9H2,2-5H3
UWKAYLJWKGQEPM-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
- Evaluation and estimation of diuretic activity of the linalyl acetate in the rats.: Focuses on the pharmacological effects of linalyl acetate, testing its diuretic activity in rats, which could lead to new therapeutic applications in medicine (Rafique et al., 2024).
- Characterization of bergamot essential oil: chemical, microbiological and colloidal aspects.: Investigates the components of bergamot essential oil, including linalyl acetate, assessing its chemical properties and potential microbiological uses, supporting its diverse applications in cosmetics and therapeutics (Cordeiro et al., 2024).
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1B
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
201.2 °F - closed cup
Punto d’infiammabilità (°C)
94 °C - closed cup
Dispositivi di protezione individuale
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W263613-100G | |
| W263613-1KG-K | 4061837877063 |
| W263613-SAMPLE | |
| W263613-SAMPLE-K | 4061837877070 |
| W263613-100G-K | 4061837877056 |
| W263613-1KG | |
| W263613-4KG | |
| W263613-4KG-K |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.