51782
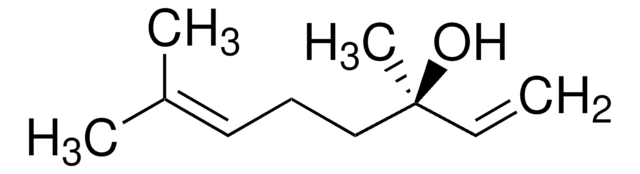
Linalool
analytical standard
Sinonimo/i:
(±)-3,7-Dimethyl-1,6-octadien-3-ol, (±)-3,7-Dimethyl-3-hydroxy-1,6-octadiene
About This Item
Prodotti consigliati
Grado
analytical standard
Livello qualitativo
Tensione di vapore
0.17 mmHg ( 25 °C)
Saggio
≥99.0% (GC)
Durata
limited shelf life, expiry date on the label
tecniche
HPLC: suitable
gas chromatography (GC): suitable
Indice di rifrazione
n20/D 1.462 (lit.)
P. eboll.
194-197 °C/720 mmHg (lit.)
Densità
0.87 g/mL at 25 °C (lit.)
applicazioni
cleaning products
cosmetics
environmental
flavors and fragrances
food and beverages
personal care
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
C\C(C)=C\CCC(C)(O)C=C
InChI
1S/C10H18O/c1-5-10(4,11)8-6-7-9(2)3/h5,7,11H,1,6,8H2,2-4H3
CDOSHBSSFJOMGT-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
171.0 °F - Pensky-Martens closed cup
Punto d’infiammabilità (°C)
77.2 °C - Pensky-Martens closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
-Cymene; Linalool; Menthol; α-Terpineol; Menthyl acetate
Protocolli
-(+)-Limonene, purum, ≥98.0% (sum of enantiomers, GC); Geranyl tiglate; α-Terpineol, natural, ≥96%, FCC, FG; Geranyl formate; α-Pinene
-Pinocarveol; Menthol; (+)-Terpinen-4-ol; α-Terpineol; (±)-α-Terpinyl acetate, predominantly α-isomer; Germacrene D
-Cymene; (−)-Menthone; α-Terpineol, natural, ≥96%, FCC, FG; Terpinolene; β-Bourbonene; 1-Octen-3-ol; β-Caryophyllene; Linalool; α-Terpinene; (−)-Menthol
Cymene; 4,5,6,7-Tetrahydro-3,6-dimethylbenzofuran; Linalool; Menthol; Menthone; Menthyl acetate; Germacrene D; Bicyclogermacrene; Thymol
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.