I5605
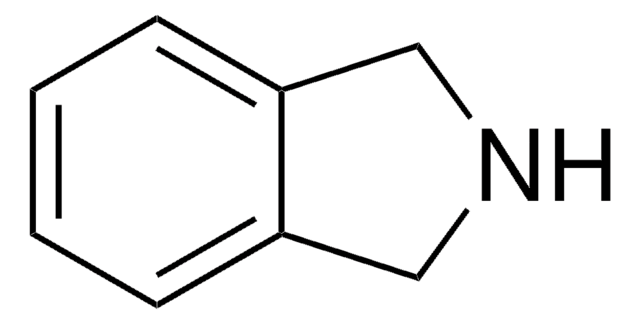
Indoline
ReagentPlus®, 99%
Sinonimo/i:
1-Azaindan, 2,3-Dihydroindole
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
C8H9N
Numero CAS:
Peso molecolare:
119.16
Beilstein:
111915
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Nome Commerciale
ReagentPlus®
Saggio
99%
Forma fisica
liquid
Indice di rifrazione
n20/D 1.592 (lit.)
P. eboll.
220-221 °C (lit.)
Densità
1.063 g/mL at 25 °C (lit.)
Stringa SMILE
C1Cc2ccccc2N1
InChI
1S/C8H9N/c1-2-4-8-7(3-1)5-6-9-8/h1-4,9H,5-6H2
LPAGFVYQRIESJQ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Reactant for preparation of:
- Inhibitors of NOD1-Induced Nuclear Factor-κB Activation
- Sphingosine-1-phosphate 4(S1P4) receptor antagonists
- Cytotoxic cell cycle inhibitors
- 2-Aminopyridines
- PET agent for imaging of protein kinase C (PKC)
- Sodium-dependent glucose co-transporter 2 (SGLT2) inhibitors for the management of hyperglycemia in diabetes
- α4β2-Nicotinic acetylcholine receptor-selective partial agonists
- mGlu4 positive allosteric modulators
- Bacterial biofilm inhibitors
- Serotonin 5-HT6 receptor antagonists
Note legali
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
199.4 °F - closed cup
Punto d’infiammabilità (°C)
93 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Matej Baláž et al.
Molecules (Basel, Switzerland), 24(18) (2019-09-22)
Performing solution-phase oximation reactions with hydroxylamine hydrochloride (NH2OH·HCl) carries significant risk, especially in aqueous solutions. In the present study, four N-substituted indole-3-carboxaldehyde oximes were prepared from the corresponding aldehydes by solvent-free reaction with NH2OH·HCl and a base (NaOH or Na2CO3)
Toshiharu Noji et al.
Organic letters, 15(8), 1946-1949 (2013-04-04)
A benzyne-mediated synthesis of substituted indolines and carbazoles was developed. The reaction includes generation of benzyne using Mg(TMP)2·2LiCl as a base, cyclization, and trapping the resulting organomagnesium intermediate with an electrophile to provide a series of substituted indolines and carbazoles
Gang He et al.
Organic letters, 14(12), 2944-2947 (2012-06-08)
An efficient method has been developed for the synthesis of indoline compounds from picolinamide (PA)-protected β-arylethylamine substrates via palladium-catalyzed intramolecular amination of ortho-C(sp(2))-H bonds. These reactions feature high efficiency, low catalyst loadings, mild operating conditions, and the use of inexpensive
Oktay Talaz et al.
Bioorganic & medicinal chemistry, 21(6), 1477-1482 (2012-11-06)
Several 1,4-bis(indolin-1-ylmethyl)benzene-based compounds containing substituents such as five, six and seven cyclic derivatives on indeno part (9a-c) were prepared and tested against two members of the pH regulatory enzyme family, carbonic anhydrase (CA). The inhibitory potencies of the compounds at
François Brucelle et al.
Organic letters, 14(12), 3048-3051 (2012-06-01)
A simple approach to prepare indolines and benzopyrrolizidinones from ortho-azidoallylbenzenes via a tandem radical addition/cyclization is described. The use of triethylborane to initiate and sustain the process provides the best results. Indolines are easily converted into the corresponding indoles by
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.