B3253
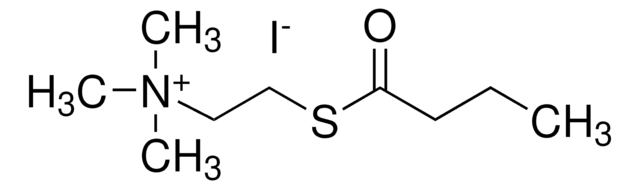
Butirriltiocolina ioduro
≥98%
Sinonimo/i:
(2-mercaptoetil)trimetilammonio ioduro butirrato
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(CH3)3N(I)CH2CH2SCOCH2CH2CH3
Numero CAS:
Peso molecolare:
317.23
Beilstein:
3729509
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
≥98%
Stato
powder
Punto di fusione
171-174 °C (lit.)
Temperatura di conservazione
−20°C
Stringa SMILE
[I-].CCCC(=O)SCC[N+](C)(C)C
InChI
1S/C9H20NOS.HI/c1-5-6-9(11)12-8-7-10(2,3)4;/h5-8H2,1-4H3;1H/q+1;/p-1
WEQAAFZDJROSBF-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Butyrylthiocholine iodide is a sulfur-containing analog of butyrylcholine. It is used as a reagent for the determination of butyrylcholinesterase activity.
Applicazioni
- Label-Free and Ultrasensitive Detection of Butyrylcholinesterase: A study demonstrated the use of Mn(II)-based electron spin resonance spectroscopy for the ultrasensitive detection of butyrylcholinesterase, using Butyrylthiocholine iodide as a substrate to quantify enzyme activity in the presence of organophosphorus pesticides, crucial for biochemical assay applications (Tang et al., 2022).
- Novel Nanozyme for Biosensing: Research developed a Co, N co-doped porous carbon-based nanozyme, demonstrating its utility as an oxidase mimic for fluorescence and colorimetric biosensing of butyrylcholinesterase, employing Butyrylthiocholine iodide as a key substrate, relevant in enzyme kinetics analysis (Sun et al., 2022).
- Detection System for Anti-Alzheimer′s Drug Screening: A fluorescent platform was constructed using copper nanoclusters and MnO2 nanosheets for the detection of butyrylcholinesterase activity, utilizing Butyrylthiocholine iodide, which may facilitate the screening of anti-Alzheimer′s drugs and probe cholinergic system interactions (Chen et al., 2022).
- Dual-Channel Detection of Butyrylcholinesterase: A study introduced bifunctional metal-organic frameworks with integrated fluorescence and oxidase activities, developed for dual-channel detection of butyrylcholinesterase using Butyrylthiocholine iodide, enhancing methodologies in biochemical assays (Wang et al., 2022).
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Ganiyu Oboh et al.
Neurotoxicology, 77, 12-19 (2019-12-15)
Many plant foods are rich sources of rutin, a flavonoid with many biological activities and health benefits. Exposure to cadmium has been implicated in neurotoxicity and cognitive dysfunction in animal models. However, there is a dearth of information on the
Cristobal Narvaez et al.
Chemosphere, 135, 75-82 (2015-04-29)
Inhibition of blood esterase activities by organophosphate (OP) pesticides has been used as a sensitive biomarker in birds. Furthermore, compared to mammalian vertebrates, less is known about the role of these enzyme activities in the digestive tracts of non-mammalian vertebrates
Gabriela Fernandes et al.
The journal of adhesive dentistry, 22(3), 265-274 (2020-05-22)
To investigate whether dental adhesives modified with polyacrylic acid copper iodide particles could inhibit esterase activity in vitro and the copper release rate from resin matrices, as well as the correlation between the two variables. Different concentrations of copper iodide
Oya Unsal-Tan et al.
MedChemComm, 10(6), 1018-1026 (2019-07-16)
A novel series of 2-pyrazoline derivatives were designed, synthesized, and evaluated for cholinesterase (ChE) inhibitory, Aβ anti-aggregating and neuroprotective activities. Among these, 3d, 3e, 3g, and 3h were established as the most potent and selective BChE inhibitors (IC50 = 0.5-3.9
Gabriele Horn et al.
Archives of toxicology, 89(3), 405-414 (2014-06-11)
Organophosphorus compounds (OP) are bound to human butyrylcholinesterase (BChE) and endogenous or exogenous BChE may act as a stoichiometric scavenger. Adequate amounts of BChE are required to minimize toxic OP effects. Simultaneous administration of BChE and oximes may transfer the
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.