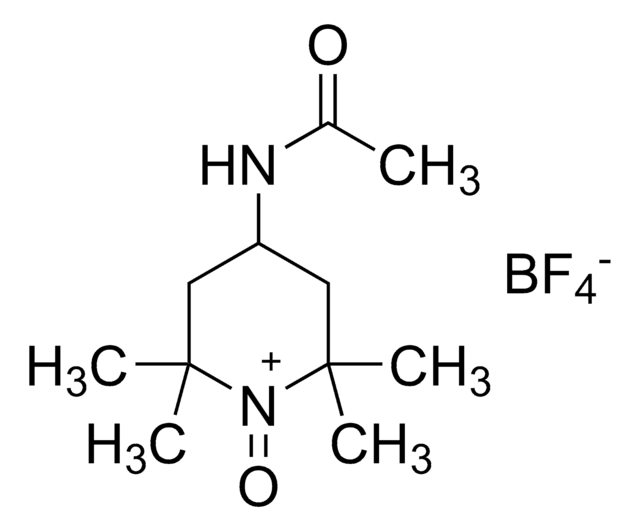
701718
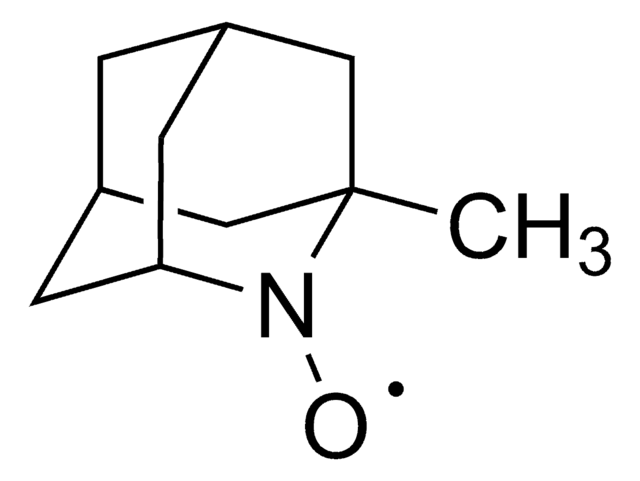
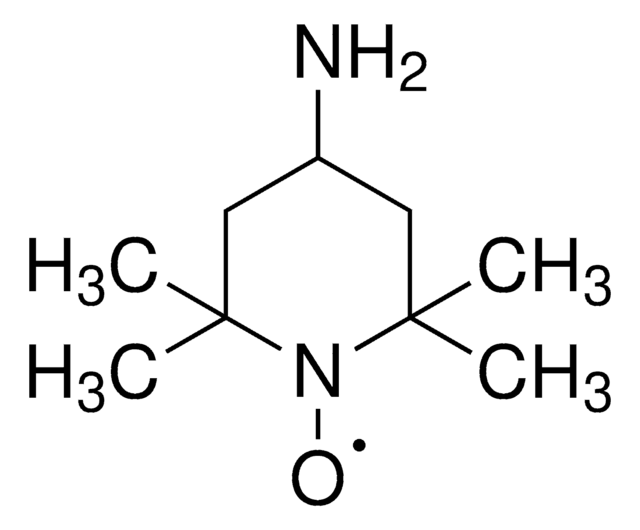
2-Azaadamantane-N-oxyl
90%
Sinonimo/i:
AZADO
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
90%
Stato
powder
Impiego in reazioni chimiche
reagent type: oxidant
Punto di fusione
182-189 °C (D)
Temperatura di conservazione
2-8°C
Stringa SMILE
[O]N1[C@@H]2C[C@H]3C[C@@H](C2)C[C@@H]1C3
InChI
1S/C9H14NO/c11-10-8-2-6-1-7(4-8)5-9(10)3-6/h6-9H,1-5H2/t6-,7+,8-,9+
BCJCJALHNXSXKE-SPJNRGJMSA-N
Categorie correlate
Descrizione generale
Applicazioni
- As catalyst for the oxidation of wood cellulose.
- As catalyst in the total synthesis of Yaku′amide A, a potential cytotoxin obtained from sponge Ceratopsion sp.
- As oxidant for the oxidation of (S)-glycidol.
Avvertenze
Warning
Indicazioni di pericolo
Classi di pericolo
Acute Tox. 4 Oral
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)