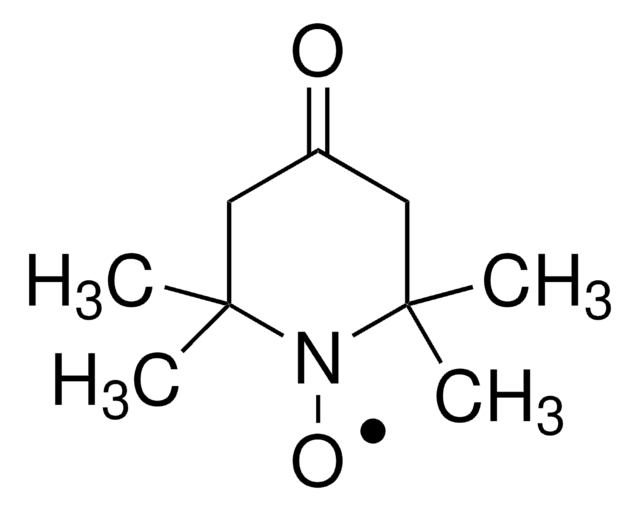
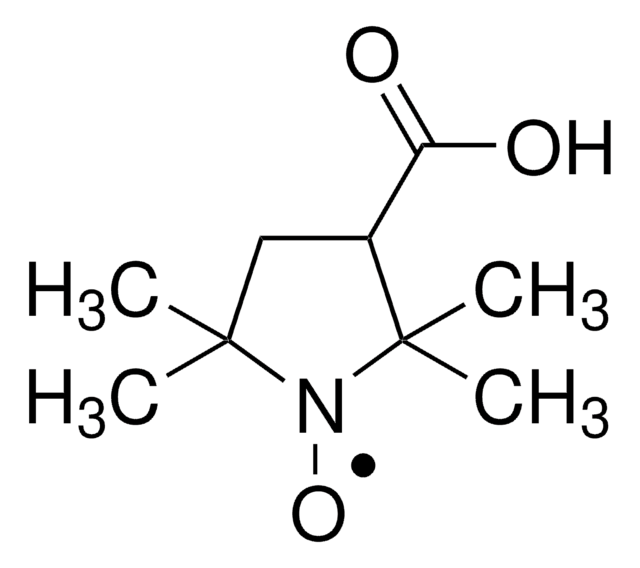
371343
4-Hydroxy-TEMPO benzoate, free radical
97%
Sinonimo/i:
4-Hydroxy-TEMPO benzoate, 4-Hydroxy-2,2,6,6-tetramethylpiperidine 1-oxyl benzoate
About This Item
Prodotti consigliati
Saggio
97%
Stato
solid
Punto di fusione
99-101 °C (lit.)
Gruppo funzionale
ester
phenyl
Stringa SMILE
CC1(C)CC(CC(C)(C)N1[O])OC(=O)c2ccccc2
InChI
1S/C16H22NO3/c1-15(2)10-13(11-16(3,4)17(15)19)20-14(18)12-8-6-5-7-9-12/h5-9,13H,10-11H2,1-4H3
MJEDTBDGYVATPI-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
TEMPO (2,2,6,6-Tetramethylpiperidinyloxy or 2,2,6,6-Tetramethylpiperidine 1-oxyl) and its derivatives are stable nitroxy radicals used as catalysts in organic oxidation reactions. TEMPO was discovered by Lebedev and Kazarnovskii in 1960. The stable free radical nature of TEMPO is due to the presence of bulky substituent groups, which hinder the reaction of the free radical with other molecules.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.