556327
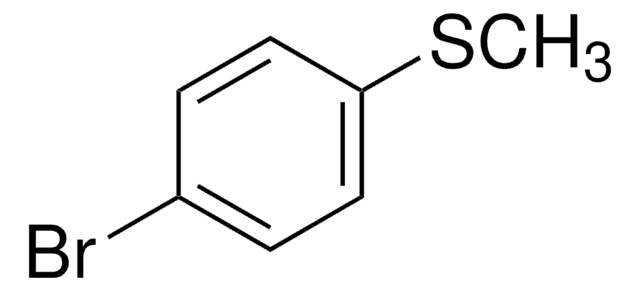
4-Bromophenyl methyl sulfone
97%
Sinonimo/i:
1-Bromo-4-(methylsulfonyl)benzene
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
BrC6H4SO2CH3
Numero CAS:
Peso molecolare:
235.10
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Punto di fusione
103-107 °C (lit.)
Stringa SMILE
CS(=O)(=O)c1ccc(Br)cc1
InChI
1S/C7H7BrO2S/c1-11(9,10)7-4-2-6(8)3-5-7/h2-5H,1H3
FJLFSYRGFJDJMQ-UHFFFAOYSA-N
Applicazioni
4-Bromophenyl methyl sulfone may be used to synthesize:
4-Bromophenyl methyl sulfone (1-bromo-4-(methylsulfonyl)benzene) can undergo coupling reaction with benzene sulfonamide in the presence of copper(I)iodide to form the corresponding N-aryl sulfonamide.
- biaryl methyl sulfones
- 5-[[-4-(methylsulfonyl)phenyl]thio]thiophene-2-sulfonamide
- 1-[4-(methylsulfonyl)phenyl]-1H-pyrazole (Hmsppz)
- DuP 697 via reaction with (5-chloro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)thiophen-2-yl)trimethylsilane
4-Bromophenyl methyl sulfone (1-bromo-4-(methylsulfonyl)benzene) can undergo coupling reaction with benzene sulfonamide in the presence of copper(I)iodide to form the corresponding N-aryl sulfonamide.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Certificati d'analisi (COA)
Cerca il Certificati d'analisi (COA) digitando il numero di lotto/batch corrispondente. I numeri di lotto o di batch sono stampati sull'etichetta dei prodotti dopo la parola ‘Lotto’ o ‘Batch’.
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Cooperativity and steric hindrance: important factors in the binding of a-cyclodextrin with para-substituted aryl alkyl sulfides, sulfoxides and sulfones.
Davies DM and Deary ME.
J. Chem. Soc. Perkin Trans. II, 7, 1287-1294 (1995)
Daniel Tordera et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(26), 8597-8609 (2013-05-08)
A new approach to obtain green-emitting iridium(III) complexes is described. The synthetic approach consists of introducing a methylsulfone electron-withdrawing substituent into a 4-phenylpyrazole cyclometalating ligand in order to stabilize the highest-occupied molecular orbital (HOMO). Six new complexes have been synthesized
?Copper-catalyzed N-arylation of sulfonamides with aryl bromides under mild conditions?
Wang X, et al.
Tetrahedron Letters, 53, 7?10-7?10 (2012)
?Divergent synthesis of 2,3,5-substituted thiophenes by C-H activation/borylation/suzuki coupling?
Kallepalli.AV, et al.
Heterocycles, 80(2), 1429 - 1448 (2010)
Carol K Wada et al.
Journal of medicinal chemistry, 45(1), 219-232 (2002-01-05)
A novel series of sulfone N-formylhydroxylamines (retrohydroxamates) have been investigated as matrix metalloproteinases (MMP) inhibitors. The substitution of the ether linkage of ABT-770 (5) with a sulfone group 13a led to a substantial increase in activity against MMP-9 but was
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.