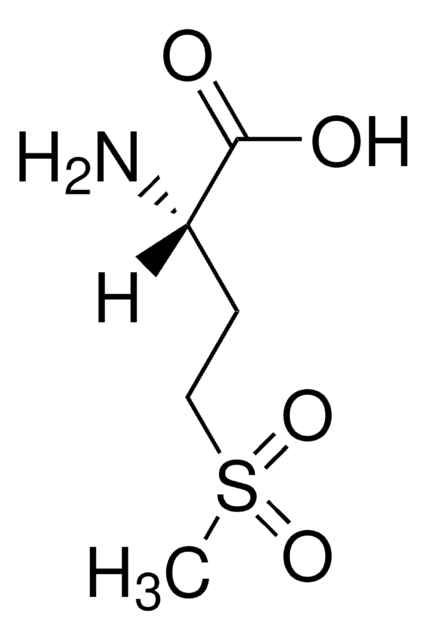
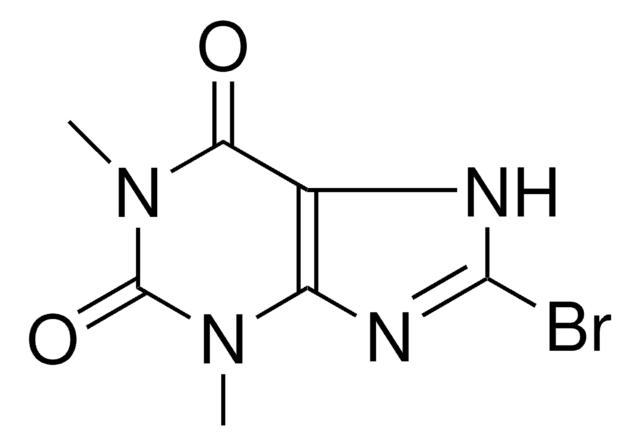
535125
2-Bromohypoxanthine
96%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula empirica (notazione di Hill):
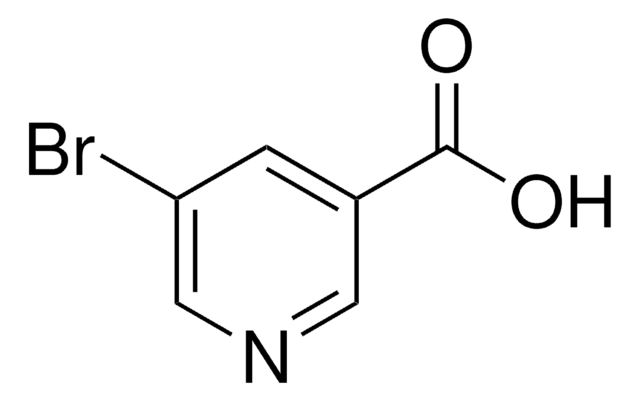
C5H3BrN4O
Numero CAS:
Peso molecolare:
215.01
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
96%
Punto di fusione
>350 °C (lit.)
Gruppo funzionale
bromo
Stringa SMILE
BrC1=Nc2nc[nH]c2C(=O)N1
InChI
1S/C5H3BrN4O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H2,7,8,9,10,11)
ONXCBJOMYNPZNI-UHFFFAOYSA-N
Categorie correlate
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
R S Sodum et al.
Chemical research in toxicology, 11(12), 1453-1459 (1998-12-22)
2-Nitropropane, an industrial chemical and a hepatocarcinogen in rats, induces aryl sulfotransferase-mediated liver DNA and RNA base modifications [Sodum, R. S., Sohn, O. S., Nie, G., and Fiala, E. S. (1994) Chem. Res. Toxicol. 7, 344-351]. Two of these modifications
H Xu et al.
Journal of medicinal chemistry, 38(1), 49-57 (1995-01-06)
Two series of selective inhibitors of herpes simplex virus types 1 and 2 (HSV1,2) thymidine kinases (TK) have been developed as potential treatment of recurrent virus infections. Among compounds related to the potent base analog N2-[m-(trifluoromethyl)phenyl]guanine (mCF3-PG), none was a
Sheng Ding et al.
Journal of combinatorial chemistry, 4(2), 183-186 (2002-03-12)
A resin-capture and release strategy for making combinatorial 2,6,9-trisubstituted purine libraries is demonstrated by capturing N9-derivatized purines at the C6 position with a thio-modified polymer. The C2 fluoro group is subsequently substituted with primary and secondary amines followed by thioether
M M Butler et al.
Nucleic acids research, 18(24), 7381-7387 (1990-12-25)
6-(p-Hydroxyphenylhydrazino)uracil (H2-HPUra) is a selective and potent inhibitor of the replication-specific class III DNA polymerase (pol III) of Gr+ bacteria. Although formally a pyrimidine, H2-HPUra derives its inhibitory activity from its specific capacity to mimic the purine nucleotide, dGTP. We
Andrzej Manikowski et al.
Journal of medicinal chemistry, 48(11), 3919-3929 (2005-05-27)
Derivatives of the herpes simplex thymidine kinase inhibitor HBPG [2-phenylamino-9-(4-hydroxybutyl)-6-oxopurine] have been synthesized and tested for inhibitory activity against recombinant enzymes (TK) from herpes simplex types 1 and 2 (HSV-1, HSV-2). The compounds inhibited phosphorylation of [3H]thymidine by both enzymes
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.