530182
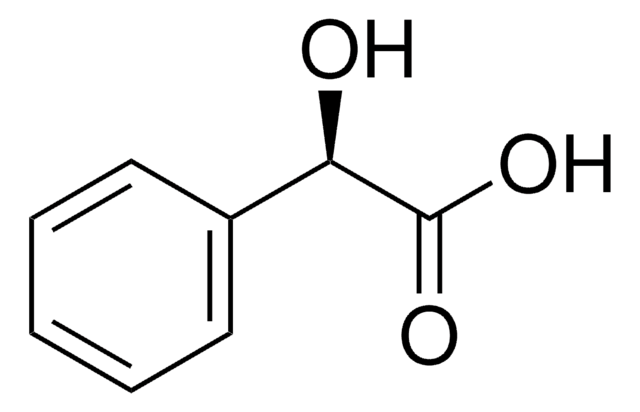
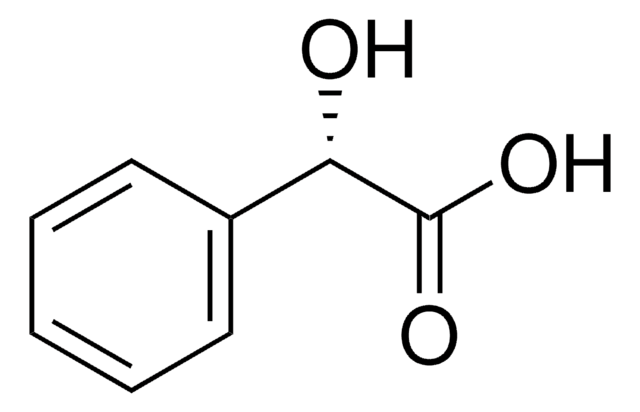
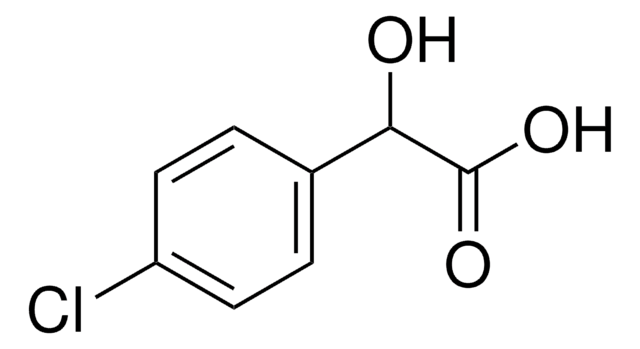
2-Chloromandelic acid
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
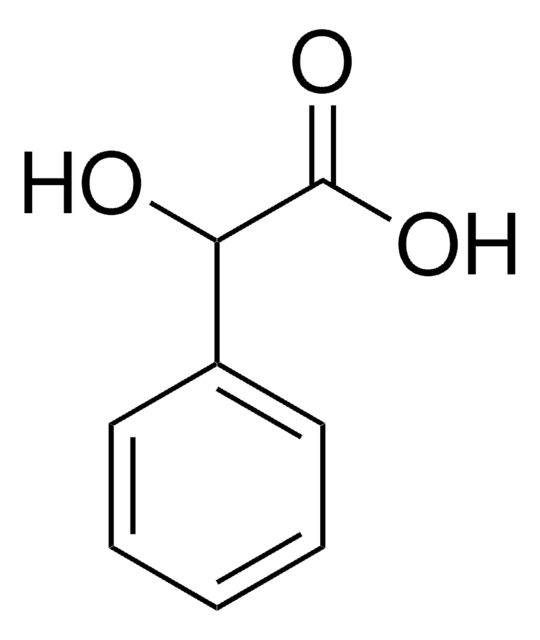
ClC6H4CH(OH)CO2H
Numero CAS:
Peso molecolare:
186.59
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
90-93 °C (lit.)
Gruppo funzionale
carboxylic acid
chloro
hydroxyl
Stringa SMILE
OC(C(O)=O)c1ccccc1Cl
InChI
1S/C8H7ClO3/c9-6-4-2-1-3-5(6)7(10)8(11)12/h1-4,7,10H,(H,11,12)
RWOLDZZTBNYTMS-UHFFFAOYSA-N
Descrizione generale
2-Chloromandelic acid (2-ClMA) is a mandelic acid derivative. A report based on its solid state-NMR, X-ray powder diffraction (XPRD) and Fourier transform infrared spectroscopy (FTIR) data reveals that in solid state 2-ClMA exists as a racemic compound. The study also suggests that the crystals of racemic 2-ClMA belongs the monoclinic space group P21/c. The efficiency of (R)-(+)-N-benzyl-1-phenylethylamine in resolving racemate 2-ClMA has been investigated.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Quan He et al.
Journal of pharmaceutical sciences, 98(5), 1835-1844 (2008-10-07)
The racemate and enantiomers of 2-chloromandelic acid were characterized by SS-NMR, XRPD, and FTIR. The binary melting point phase diagram was constructed by DSC (differential scanning calorimetry). The solid-state nature of 2-chloromandelic acid was identified to be a racemic compound.
Xin Yuan et al.
Biotechnology progress, 35(4), e2815-e2815 (2019-04-10)
Optically pure 2-chloromandelic acid (ClMA) is a very important chiral drug intermediate for synthesis of (S)-clopidogrel, belonging to the platelet aggregation inhibitor. Enantioselective resolution of (R,S)-2-chloromandelic acid was carried out in organic solvent through irreversible transesterification catalyzed by lipase AK
Yangfeng Peng et al.
Chirality, 24(5), 349-355 (2012-04-18)
During the resolution of 2-chloromandelic acid with (R)-(+)-N-benzyl-1-phenylethylamine, the crystals of the less soluble salt were grown, and their structure were determined and presented. The chiral discrimination mechanism was investigated by examining the weak intermolecular interactions (such as hydrogen bond
Shengqiang Tong et al.
Journal of separation science, 38(12), 2085-2092 (2015-04-14)
The chromatographic retention mechanism describing relationship between retention factor and concentration of Cu(2+) (l-phenylalanine)2 using chiral ligand mobile phase was investigated and eight mandelic acid derivatives were enantioseparated by chiral ligand exchange chromatography. The relationship between retention factor and concentration
Rafael Gozalbes et al.
Bioorganic & medicinal chemistry, 18(19), 7078-7084 (2010-09-03)
Solubility plays a very important role in the selection of compounds for drug screening. In this context, a QSAR model was developed for predicting water solubility of drug-like compounds. First, a set of relevant parameters for establishing a drug-like chemical
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.