418080
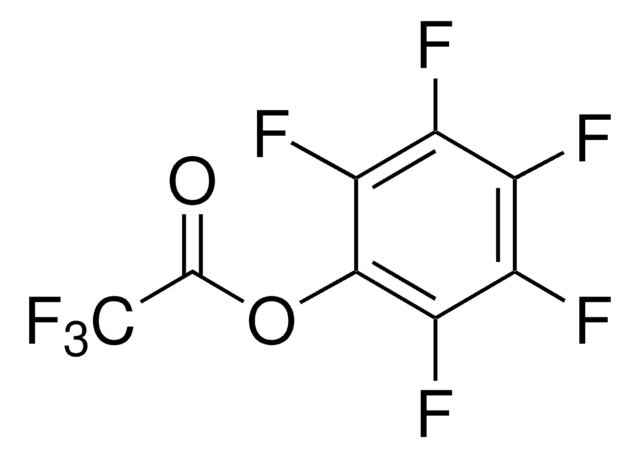
Pentafluorophenyl diphenylphosphinate
Sinonimo/i:
FDPP, Diphenylphosphinic acid pentafluorophenyl ester
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
(C6H5)2P(O)OC6F5
Numero CAS:
Peso molecolare:
384.24
Numero MDL:
Codice UNSPSC:
12352101
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Stato
solid
Livello qualitativo
Punto di fusione
47-50 °C (lit.)
Gruppo funzionale
fluoro
Stringa SMILE
Fc1c(F)c(F)c(OP(=O)(c2ccccc2)c3ccccc3)c(F)c1F
InChI
1S/C18H10F5O2P/c19-13-14(20)16(22)18(17(23)15(13)21)25-26(24,11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10H
OOWSDKUFKGVADH-UHFFFAOYSA-N
Categorie correlate
Descrizione generale
Pentafluorophenyl diphenylphosphinate (FDPP) is a reagent, used as a coupling agent in the amide bond forming reactions without racemization. It can also be employed in the synthesis of dipeptides with high yields and good optical purity. FDPP can be prepared from diphenylphosphinic chloride and pentafluorophenol in the presence of imidazole.
Applicazioni
Catalyst involved in coupling and macrocyclization
Reagent used in:
Reagent used in:
- Fmoc solid-phase synthesis
- Synthesis of phorboxazoles, furan-based cyclic homoligopeptides, and ziziphine N
FDPP can be used as a coupling reagent in the:
- Synthesis of peptides by solid-phase and solution-phase reactions.
- Preparation of macrocyclic peptide cyclotheonamide B as a thrombin inhibitor.
- Cyclooligomerization of N-methylated-L-valine thiazole amino acid to obtain its cyclic tetramers.
- Macrocyclization of an intermediate for the total synthesis of ziziphine N , and for the macrolactamization of precursor in synthesizing cryptophycin D.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Highly convergent route to cyclopeptide alkaloids. Total synthesis of ziziphine N.
He G, et al.
Organic Letters, 9(7), 1367-1369 (2007)
Synthesis of novel N-methylated thiazole-based cyclic octa-and dodecapeptides.
Dudin L, et al.
Tetrahedron, 61(5), 1257-1267 (2005)
Total synthesis of cryptophycins. Revision of the structures of cryptophycins A and C.
Barrow R A, et al.
Journal of the American Chemical Society, 117(9), 2479-2490 (1995)
Pentafluorophenyl diphenylphosphinate a new efficient coupling reagent in peptide chemistry.
Chen S and Xu J
Tetrahedron Letters, 32(46), 6711-6714 (1991)
Gang He et al.
Organic letters, 9(7), 1367-1369 (2007-03-10)
[structure: see text]. A highly convergent protocol to cyclopeptide alkaloids, as demonstrated by the first total synthesis of antiplasmodial agent ziziphine N, is developed. The key elements include construction of its aryl ether unit via Mitsunobu reaction, installation of its
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.



![Sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate](/deepweb/assets/sigmaaldrich/product/structures/251/439/7a621e74-bfd1-4a43-833c-09adfcc1e0b3/640/7a621e74-bfd1-4a43-833c-09adfcc1e0b3.png)