40766
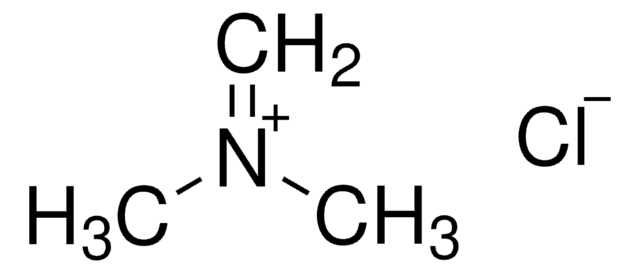
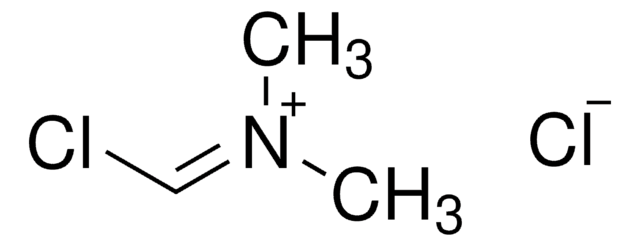
N,N-Dimethylmethyleneiminium chloride
≥95.0% (AT)
Sinonimo/i:
Böhme′s salt, Böhme′s salt, Dimethylformiminium chloride, Methylenedimethylammonium chloride
About This Item
Prodotti consigliati
Livello qualitativo
Saggio
≥95.0% (AT)
Stato
powder
Impiego in reazioni chimiche
reaction type: C-C Bond Formation
Punto di fusione
146-148 °C (lit.)
Gruppo funzionale
amine
Stringa SMILE
[Cl-].C[N+](C)=C
InChI
1S/C3H8N.ClH/c1-4(2)3;/h1H2,2-3H3;1H/q+1;/p-1
ZJTROANVDZIEGB-UHFFFAOYSA-M
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Applicazioni
- Electrophilic aminomethylation of aldehydes and ketones to synthesize corresponding Mannich products, also known as Mannich reaction.
- Preparation of dihydronaphthyridinones as HIV-1 integrase inhibitors.
- Preparation of cyanotetrahydrooxo-β-carbolines via cyclocondensation of cyanomethyl indole-2-carboxylate with ammonia.
- Asymmetric synthesis of phalarine via stereospecific Pictet-Spengler cyclocondensation and traceless chirality transfer from L-tryptophan.
- Synthesis of functionalized diarylpyrrolizines as inhibitors of microsomal prostaglandin E2 synthase-1 (mPGES-1) and 5-lipoxygenase (5-LOX).
- Synthesis of (±)-phalarine with the rearrangement of an azaspiroindolenine and Gassman oxindole preparation as key steps.
Preparation of dihydronaphthyridinones as HIV-1 integrase inhibitors
Mannich reactions
Preparation of cyanotetrahydrooxo-ß-carbolines via cyclocondensation reactions
Asymmetric synthesis of phalarine via stereospecific Pictet-Spengler cyclocondensation and traceless chirality transfer from L-tryptophan
Synthesis of functionalized diarylpyrrolizines as inhibitors of microsomal prostaglandin E2 synthase-1 (mPGES-1) and 5-lipoxygenase (5-LOX)
Synthesis of (±)-phalarine with the rearrangement of an azaspiroindolenine and Gassman oxindole preparation as key steps
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Flam. Sol. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
4.1B - Flammable solid hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
179.6 °F - closed cup
Punto d’infiammabilità (°C)
82 °C - closed cup
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.