407097
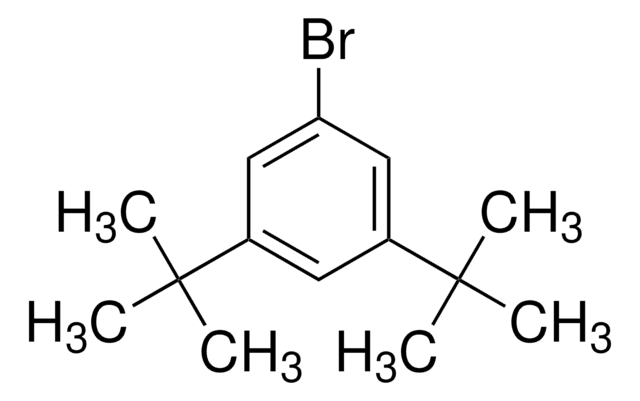
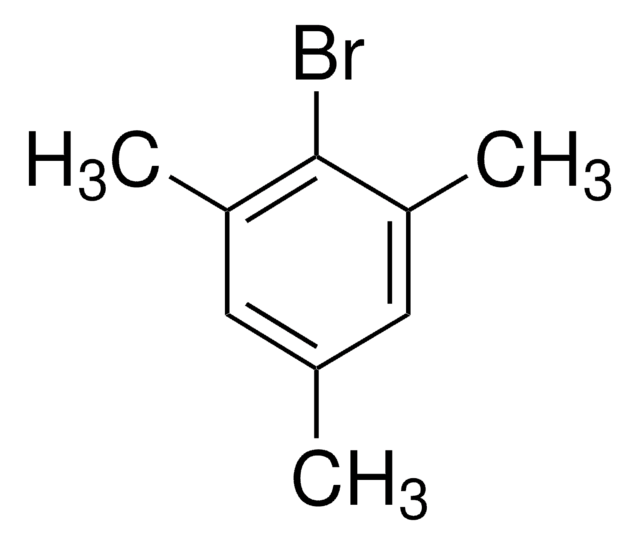
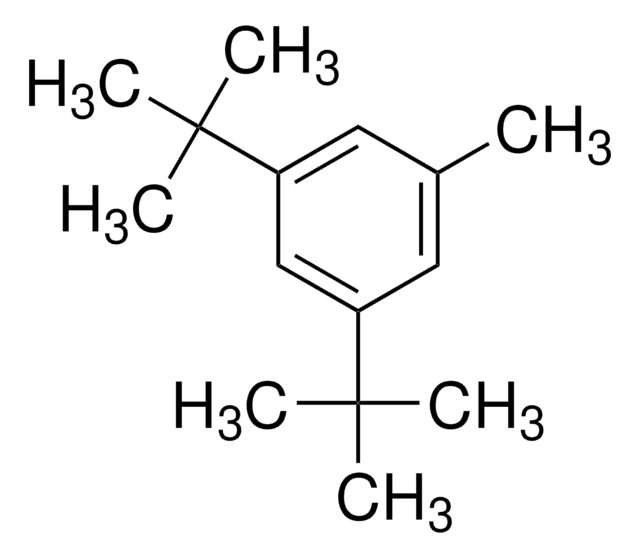
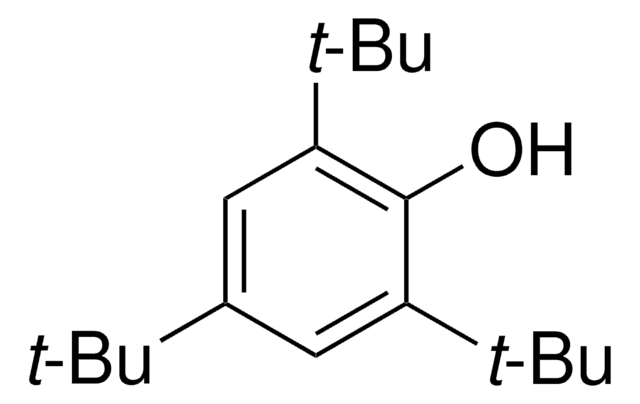
1-Bromo-2,4,6-tri-tert-butylbenzene
97%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
[(CH3)3C]3C6H2Br
Numero CAS:
Peso molecolare:
325.33
Beilstein:
1913257
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
97%
Punto di fusione
168-173 °C (lit.)
Gruppo funzionale
bromo
Stringa SMILE
CC(C)(C)c1cc(c(Br)c(c1)C(C)(C)C)C(C)(C)C
InChI
1S/C18H29Br/c1-16(2,3)12-10-13(17(4,5)6)15(19)14(11-12)18(7,8)9/h10-11H,1-9H3
JOKZWHPYNRDCOA-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
1-Bromo-2,4,6-tri-tert-butylbenzene (2,4,6-tri-tert-butylbromobenzene) is a hindered aryl bromide. 1-Bromo-2,4,6-tri-tert-butylbenzene on reaction with phenylboronic acid yields α,α-dimethyl-β-phenyl hydrostyrene.
Applicazioni
1-Bromo-2,4,6-tri-tert-butylbenzene was used in the synthesis of bulky biarylphosphine ligand. This ligand was reported to participate in the Pd-catalyzed C-O cross-coupling of a wide range of aryl halides and phenols under milder conditions. It was used to investigate the effect on oligomerization of increased steric bulk in dimethylindium(III) chalcogenolates. It may be used to form α,α-dimethyl-β-phenyl hydrostyrene by reacting with phenylboronic acid.
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
The first Cu-and amine-free Sonogashira-type cross-coupling in the C-6-alkynylation of protected 2'-deoxyadenosine.
Ngassa FN, et al.
Tetrahedron, 65(21), 4085-4091 (2009)
Luca Salvi et al.
Organic letters, 14(1), 170-173 (2011-12-21)
A new bulky biarylphosphine ligand (L8) has been developed that allows the Pd-catalyzed C-O cross-coupling of a wide range of aryl halides and phenols under milder conditions than previously possible. A direct correlation between the size of the ligand substituents
Palladium(0)-catalyzed intermolecular amination of unactivated C(sp³)-H bonds.
Jun Pan et al.
Angewandte Chemie (International ed. in English), 50(37), 8647-8651 (2011-08-04)
Glen G Briand et al.
Dalton transactions (Cambridge, England : 2003), 39(16), 3833-3841 (2010-04-08)
The effect on oligomerization of increased steric bulk in dimethylindium(III) chalcogenolates (Me(2)InER') (E = O, S, Se) has been examined. The facile reaction of Me(3)In with a series of phenols, thiophenols and selenophenols afforded the compounds [Me(2)InO(C(6)H(5))](2) (1), [Me(2)InO(2,6-Me(2)C(6)H(3))](2) (2)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.