37760
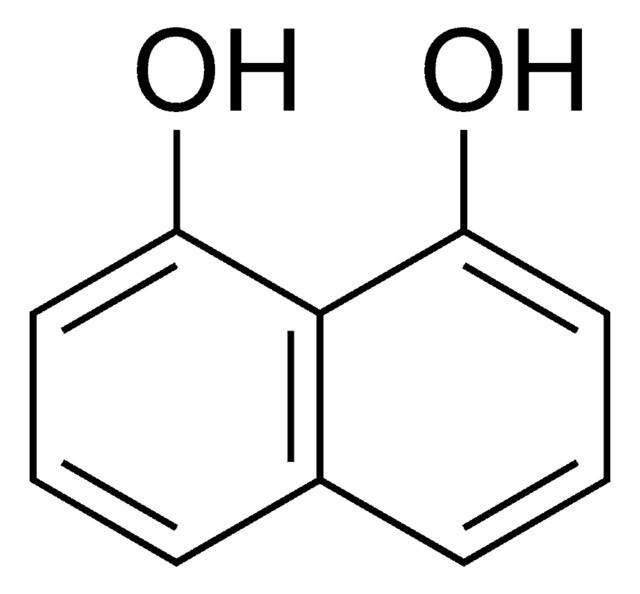
2,3-Dihydroxynaphthalene
≥98.0% (HPLC)
Sinonimo/i:
2,3-Naphthalenediol
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C10H6(OH)2
Numero CAS:
Peso molecolare:
160.17
Beilstein:
742375
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Livello qualitativo
Saggio
≥98.0% (HPLC)
Residuo dopo sublimazione
≤1%
Punto di fusione
161-165 °C (lit.)
162-164 °C
Stringa SMILE
Oc1cc2ccccc2cc1O
InChI
1S/C10H8O2/c11-9-5-7-3-1-2-4-8(7)6-10(9)12/h1-6,11-12H
JRNGUTKWMSBIBF-UHFFFAOYSA-N
Informazioni sul gene
human ... BAD(572)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
2,3-Dihydroxynaphthalene is a polyhydroxy phenol. It is an aromatic dihydroxy compound having hydroxyl groups at ortho positions. Its reaction with molybdenum(VI) complexes has been reported. The asymmetric oxidative coupling polymerization of 2,3-dihydroxynaphthalene using the Cu(I)-bisoxazoline complex as catalyst has been reported to afford poly(2,3-dihydroxy-1,4-naphthylene), having a continuous 1,1′-bi-2-naphthol main chain structure. The nitrodisplacement reaction between nitrophthalodinitriles and 2,3-dihydroxynaphthalene has been investigated.
Applicazioni
2,3-Dihydroxynaphthalene may be used in the following studies:
- Construction of dinaphtho[2,1-b;2′,3′-d]furan-6-ol, via dehydration reaction in the presence of strong acid.
- As fused ring catecholate type ligand for the surface modification of nanocrystalline TiO2 particles.
- As adsorptive and competing ligand during the chemical speciation of iron in seawater by cathodic stripping voltammetry.
- Synthesis of cyclotriphosphazene derivatives, used as non-halogen flame retardants
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Eye Dam. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
347.0 °F
Punto d’infiammabilità (°C)
175 °C
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Application of cyclophosphazene derivatives as flame retardants for ABS.
Shin YJ, et al.
Journal of Industrial and Engineering Chemistry (Amsterdam, Netherlands), 16(3), 364-367 (2010)
Chemical speciation of iron in seawater by cathodic stripping voltammetry with dihydroxynaphthalene.
Constant M G van den Berg
Analytical chemistry, 78(1), 156-163 (2005-12-31)
The chemical speciation of iron in seawater is determined by cathodic stripping voltammetry using 2,3-dihydroxynaphthalene (DHN) as adsorptive and competing ligand. The optimized conditions include a DHN concentration of 0.5-1 microM, seawater at its original pH of 8, and equilibration
Kentaro Nakanishi et al.
The Journal of organic chemistry, 79(6), 2625-2631 (2014-02-26)
The construction of dinaphtho[2,1-b;2',3'-d]furan-6-ol was developed via a dehydration reaction involving two molecules of 2,3-dihydroxynaphthalene in the presence of a strong acid. Starting from the dinaphthofuran, a variety of butterfly shaped derivatives were synthesized. The optical properties of these compounds
Tatjana D Savić et al.
Nanoscale, 4(5), 1612-1619 (2012-02-09)
Surface modification of nanocrystalline TiO(2) particles (45 Å) with catecholate-type ligands consisting of an extended aromatic ring system, i.e., 2,3-dihydroxynaphthalene and anthrarobin, was found to alter the optical properties of the nanoparticles in a similar way to modification with catechol.
Synthesis of bis (ether anhydride) s for poly (ether imide) s having 1, 2-linked units by nitrodisplacement with catechol derivatives.
Eastmond GC and Paprotny J.
Macromolecules, 28(7), 2140-2146 (1995)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.