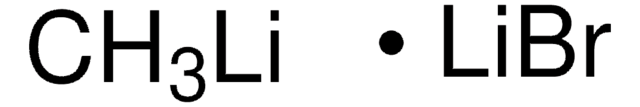197343
Methyllithium solution
1.6 M in diethyl ether
Sinonimo/i:
Lithium methanide, MeLi
About This Item
Prodotti consigliati
Densità del vapore
3 (vs air)
Livello qualitativo
Stato
liquid
Composizione
halide, ~0.05 M
Concentrazione
1.6 M in diethyl ether
Densità
0.732 g/mL at 25 °C
Temperatura di conservazione
2-8°C
Stringa SMILE
[Li]C
InChI
1S/CH3.Li/h1H3;
DVSDBMFJEQPWNO-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
Confezionamento
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Flam. Liq. 2 - Pyr. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
Rischi supp
Codice della classe di stoccaggio
4.2 - Pyrophoric and self-heating hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
1.4 °F - closed cup
Punto d’infiammabilità (°C)
-17 °C - closed cup
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Reagents for C–C Bond Formation
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 197343-18L-C | 4061837880377 |
| 197343-20L | |
| 197343-250ML | |
| 197343-4X25ML | 4061838762450 |
| 197343-500ML | |
| 197343-800ML | 4061838762467 |
| 197343-50ML | 4061837880438 |
| 197343-100ML | 4061838762436 |
| 197343-10KG | |
| 197343-18L | |
| 197343-1L | 4061837880384 |
| 197343-4X100ML | 4061838762443 |
| 197343-4X10ML | |
| 197343-8L | 4061837880445 |
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.