148229
Thiobenzamide
98%
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
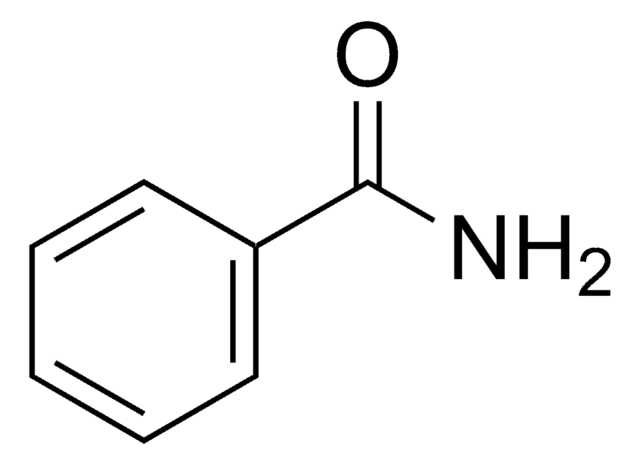
Formula condensata:
C6H5CSNH2
Numero CAS:
Peso molecolare:
137.20
Beilstein:
606021
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Punto di fusione
113-117 °C (lit.)
Stringa SMILE
NC(=S)c1ccccc1
InChI
1S/C7H7NS/c8-7(9)6-4-2-1-3-5-6/h1-5H,(H2,8,9)
QIOZLISABUUKJY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
Thiobenzamide was used to prepare amide and amidine adducts. It was also used in the synthesis of 4-oxo-4H-chromene-3-carbothioic acid N-phenylamides.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 3 Oral
Codice della classe di stoccaggio
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Yakov M Koen et al.
Chemical research in toxicology, 26(4), 564-574 (2013-03-08)
Thioacetamide (TA) has long been known as a hepatotoxicant whose bioactivation requires S-oxidation to thioacetamide S-oxide (TASO) and then to the very reactive S,S-dioxide (TASO2). The latter can tautomerize to form acylating species capable of covalently modifying cellular nucleophiles including
M M Simile et al.
Carcinogenesis, 17(7), 1533-1537 (1996-07-01)
S-Adenosyl-L-methionine (SAM) is a strong chemopreventive agent of rat liver carcinogenesis. Examination was made to determine whether inhibition by SAM of the development of preneoplastic liver lesions persists to SAM withdrawal in diethylnitrosamine-initiated F344 rats promoted with thiobenzamide (TB). The
W G Chung et al.
Molecules and cells, 7(6), 738-741 (1998-03-24)
Flavin-containing monooxygenase (FMO), known not to be induced by xenobiotics, has been induced by a polycyclic aromatic hydrocarbon, 3-methylcholanthrene (3MC). We have found a prominent augmentation of hepatic FMO1 both at transcription and translation levels by pretreatment of rats with
Presence of flavin-containing monooxygenase in rat brain.
S Bhamre et al.
Biochemical pharmacology, 42(2), 442-444 (1991-07-05)
T Mizutani et al.
Toxicology letters, 85(2), 101-105 (1996-05-01)
The hepatotoxicity of the 3 isomers of para-substituted thiobenzamides and the 3 isomers of 2-(para-substituted phenyl)-4-methylthiazoles was evaluated in mice depleted of glutathione (GSH) by pretreatment with buthionine sulfoximine (BSO). In accordance with previous studies with the rat, p-methoxythiobenzamide was
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.