113190
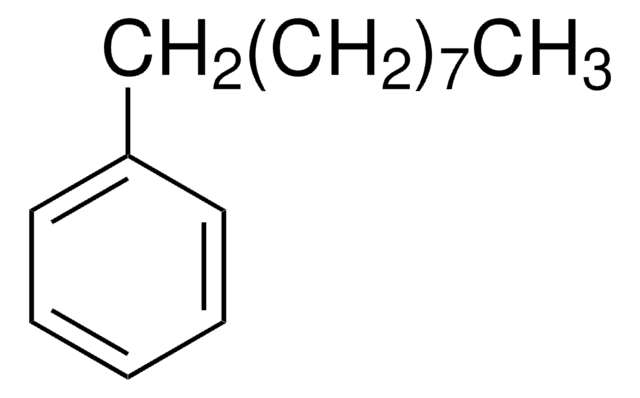
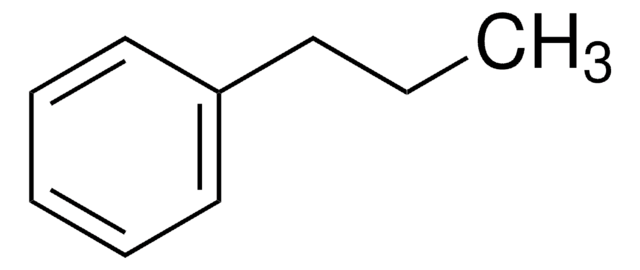
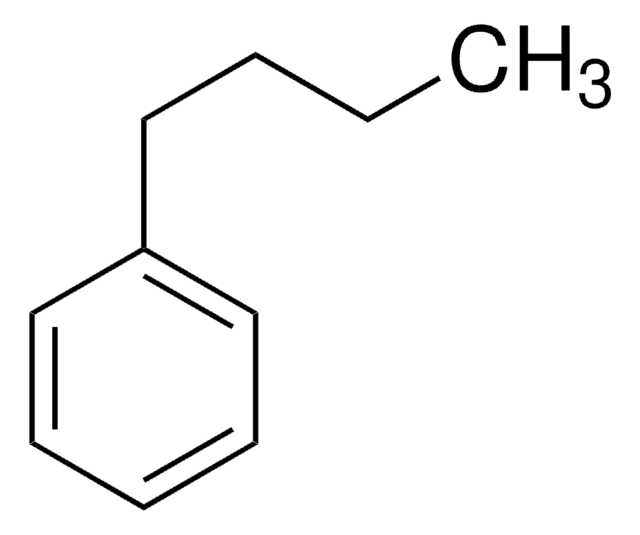
1-Phenyloctane
98%
Sinonimo/i:
Octylbenzene
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H5(CH2)7CH3
Numero CAS:
Peso molecolare:
190.32
Beilstein:
1906253
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.22
Prodotti consigliati
Saggio
98%
Indice di rifrazione
n20/D 1.484 (lit.)
P. ebollizione
261-263 °C (lit.)
Punto di fusione
−36 °C (lit.)
Densità
0.858 g/mL at 25 °C (lit.)
Gruppo funzionale
phenyl
Stringa SMILE
CCCCCCCCc1ccccc1
InChI
1S/C14H22/c1-2-3-4-5-6-8-11-14-12-9-7-10-13-14/h7,9-10,12-13H,2-6,8,11H2,1H3
CDKDZKXSXLNROY-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Octylbenzene (1-Phenyloctane) forms charge-transfer complexes with fluoranil and 1,3,5-trinitrobenzene.
Applicazioni
1-Phenyloctane is used as a solvent to study the self-assembly of derivatives of tetrathiafulvalene (TTF) on graphite and their visualization by scanning tunnelling microscopy (STM). It is also used to study the effects of the substitution of these compounds on the interactions between them.
Codice della classe di stoccaggio
10 - Combustible liquids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
224.6 °F - closed cup
Punto d’infiammabilità (°C)
107 °C - closed cup
Dispositivi di protezione individuale
Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Yanqiu Jiang et al.
Scientific reports, 5, 17720-17720 (2015-12-05)
The interface between organic semiconductor and graphene electrode, especially the structure of the first few molecular layers at the interface, is crucial for the device properties such as the charge transport in organic field effect transistors. In this work, we
Elba Gomar-Nadal et al.
Chemical communications (Cambridge, England), (7)(7), 906-907 (2003-05-13)
The synthesis, isolation and STM imaging on graphite of the cis and trans isomers of a TTF reveal isomer-dependent packing, and constitutes a way to study the non-covalent interactions at play in these systems.
Yan Yang et al.
Langmuir : the ACS journal of surfaces and colloids, 31(45), 12408-12416 (2015-10-29)
Side chains containing two diacetylene units spaced by an odd number of methylene units exhibit pronounced “bumps” composed of 0.3 nm steps, in opposite directions, at odd and even side-chain positions. In densely packed self-assembled monolayers, the bis-diacetylene bumps stack
Xiaodong Chen et al.
Nano letters, 7(11), 3483-3488 (2007-10-05)
Selective adsorption of semiconductor nanocrystals onto an organic self-organized pattern shows a time-dependent behavior. By studying the wetting behavior of delivered solvent (1-phenyloctane) on a lipid self-organized pattern and determining the adhesion energy between semiconductor nanocrystals and substrate, we obtain
Assemblies at the liquid-solid interface: chirality expression from molecular conformers.
Yong-Tao Shen et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 14(1), 92-95 (2012-11-13)
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.