Wichtige Dokumente
W253901
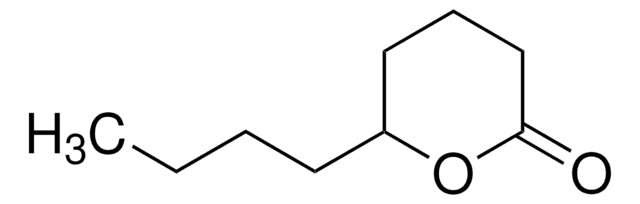
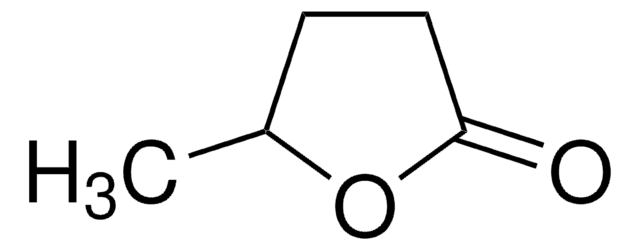
γ-Heptalacton
≥98%, FCC, FG
Synonym(e):
(±)-4-Heptanolid, (±)-γ-Propyl-γ-butyrolacton, (±)-Dihydro-5-propyl-2(3H)-furanon
About This Item
Empfohlene Produkte
Biologische Quelle
synthetic
Qualitätsniveau
Qualität
FG
Fragrance grade
Halal
Kosher
Agentur
follows IFRA guidelines
meets purity specifications of JECFA
Einhaltung gesetzlicher Vorschriften
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FCC
FDA 21 CFR 117
FDA 21 CFR 172.515
Assay
≥98%
Brechungsindex
n20/D 1.442 (lit.)
bp
61-62 °C/2 mmHg (lit.)
Dichte
0.999 g/mL at 25 °C (lit.)
Anwendung(en)
flavors and fragrances
Dokumentation
see Safety & Documentation for available documents
Nahrungsmittelallergen
no known allergens
Allergener Duftstoff
no known allergens
Organoleptisch
coconut; creamy; sweet
SMILES String
CCCC1CCC(=O)O1
InChI
1S/C7H12O2/c1-2-3-6-4-5-7(8)9-6/h6H,2-5H2,1H3
InChIKey
VLSVVMPLPMNWBH-UHFFFAOYSA-N
Angaben zum Gen
human ... CYP1A2(1544)
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Anwendung
- Enzymes with Lactonase Activity against Fungal Quorum Molecules as Effective Antifungals.: This study investigates the potential of lactonase enzymes in combating fungal infections by disrupting fungal communication systems. The findings indicate significant antifungal activity, suggesting applications in developing new antifungal treatments (Efremenko et al., 2024).
- Product study of the reactions of gamma-caprolactone and gamma-heptalactone initiated by OH radicals at 298 K and atmospheric pressure: Formation of acyl peroxynitrates (APN).: This research explores the chemical reactions of gamma-heptalactone with OH radicals, leading to the formation of acyl peroxynitrates, which are significant in atmospheric chemistry and pollution studies (Baptista et al., 2023).
- Effect of gamma-Heptalactone on the Morphology and Production of Monascus Pigments and Monacolin K in Monascus purpureus.: The paper examines how gamma-heptalactone influences the production of bioactive compounds in Monascus purpureus, with implications for food and pharmaceutical industries (Shi et al., 2022).
- RIFM fragrance ingredient safety assessment, gamma-heptalactone, CAS Registry Number 105-21-5.: This safety assessment evaluates gamma-heptalactone as a fragrance ingredient, ensuring its safe use in various consumer products (Api et al., 2019).
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Global Trade Item Number
| SKU | GTIN |
|---|---|
| W253901-100G-K | 4061837806735 |
| W253901-100G | |
| W253901-1KG | |
| W253901-1KG-K | 4061837806742 |
| W253901-5KG | |
| W253901-5KG-K | 4061837806759 |
| W253901-SAMPLE | |
| W253901-SAMPLE-K | 4061837806766 |
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.