T7402
Paclitaxel
from Taxus brevifolia, ≥95% (HPLC), powder
About This Item
Produtos recomendados
fonte biológica
Taxus brevifolia
Ensaio
≥95% (HPLC)
forma
powder
cor
white
pf
213 °C (dec.) (lit.)
solubilidade
DMSO: 50 mg/mL (can be stored frozen for several months)
methanol: soluble 50 mg/mL, clear, colorless (undergoes transesterification)
ethanol: soluble
espectro de atividade do antibiótico
viruses
Modo de ação
DNA synthesis | interferes
originador
Bristol-Myers Squibb
temperatura de armazenamento
2-8°C
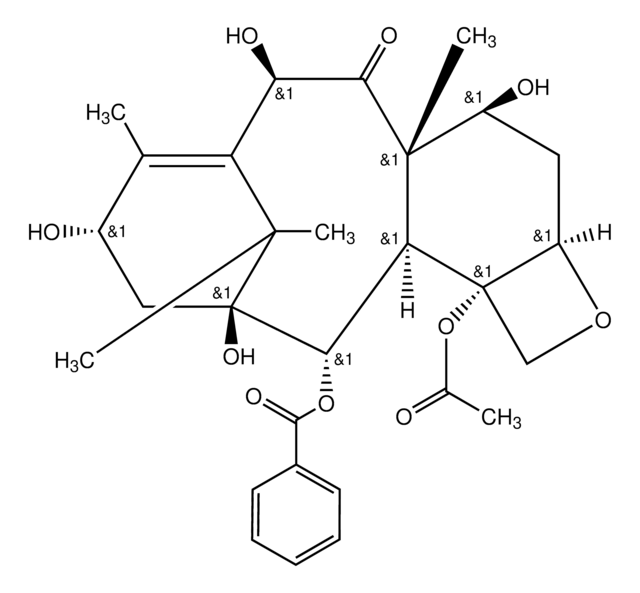
cadeia de caracteres SMILES
[H][C@@]12C[C@H](O)[C@@]3(C)C(=O)[C@H](OC(C)=O)C4=C(C)[C@H](C[C@@](O)([C@@H](OC(=O)c5ccccc5)[C@]3([H])[C@@]1(CO2)OC(C)=O)C4(C)C)OC(=O)[C@H](O)[C@@H](NC(=O)c6ccccc6)c7ccccc7
InChI
1S/C47H51NO14/c1-25-31(60-43(56)36(52)35(28-16-10-7-11-17-28)48-41(54)29-18-12-8-13-19-29)23-47(57)40(61-42(55)30-20-14-9-15-21-30)38-45(6,32(51)22-33-46(38,24-58-33)62-27(3)50)39(53)37(59-26(2)49)34(25)44(47,4)5/h7-21,31-33,35-38,40,51-52,57H,22-24H2,1-6H3,(H,48,54)/t31-,32-,33+,35-,36+,37+,38-,40-,45+,46-,47+/m0/s1
chave InChI
RCINICONZNJXQF-MZXODVADSA-N
Informações sobre genes
human ... BCL2(596) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
Características e benefícios
Embalagem
Atenção
Nota de preparo
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Muta. 2 - Repr. 1B - STOT RE 1 - STOT SE 2
Órgãos-alvo
Central nervous system,Bone marrow,Cardio-vascular system, Eyes,Central nervous system
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
We presents an article on ABC Transporters and Cancer Drug Resistance
High titer lentiviral particles including beta-actin, alpha-tubulin and vimentin used for live cell analysis of cytoskeleton structure proteins.
Conteúdo relacionado
Discover Bioactive Small Molecules for ADME/Tox
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica