M4758
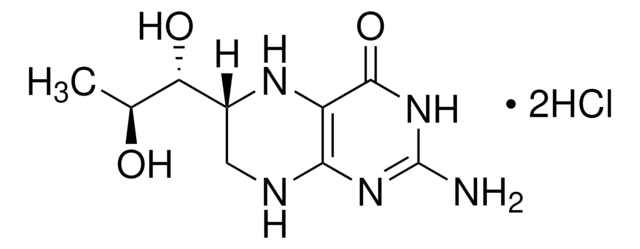
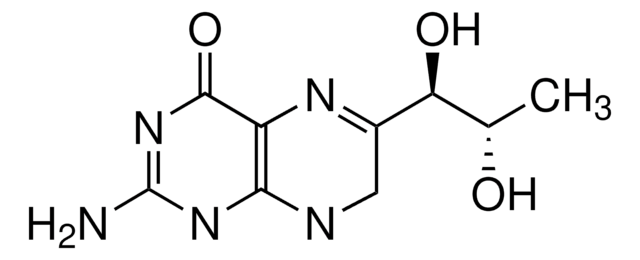
(±)-6-Methyl-5,6,7,8-tetrahydropterine dihydrochloride
~95% (TLC)
Sinônimo(s):
6-MPH4, DL-2-Amino-4-hydroxy-6-methyl-5,6,7,8-tetrahydropteridine dihydrochloride
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C7H11N5O · 2HCl
Número CAS:
Peso molecular:
254.12
Beilstein:
5648114
Número MDL:
Código UNSPSC:
12352204
ID de substância PubChem:
NACRES:
NA.51
Ensaio:
~95% (TLC)
Produtos recomendados
Ensaio
~95% (TLC)
Nível de qualidade
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
Cl.Cl.CC1CNc2nc(N)nc(O)c2N1
InChI
1S/C7H11N5O.2ClH/c1-3-2-9-5-4(10-3)6(13)12-7(8)11-5;;/h3,10H,2H2,1H3,(H4,8,9,11,12,13);2*1H
chave InChI
MKQLORLCFAZASZ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
6-MPH4 is used to study mechanisms of nitric oxide (NO) synthase and free radical induced L-DOPA release from striatal tissue.
Ações bioquímicas/fisiológicas
Synthetic cofactor for tyrosine hydrolase; also cofactor for phenylalanine and tryptophan hydroxylases; less activity than the natural cofactor, BH4
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Elizabeth A Gaskell et al.
PloS one, 4(3), e4801-e4801 (2009-03-12)
The genome of the protozoan parasite Toxoplasma gondii was found to contain two genes encoding tyrosine hydroxylase; that produces L-DOPA. The encoded enzymes metabolize phenylalanine as well as tyrosine with substrate preference for tyrosine. Thus the enzymes catabolize phenylalanine to
P Abreu-González et al.
European journal of pharmacology, 541(1-2), 33-37 (2006-06-06)
In the present study we have analyzed the effect of tetrahydrobiopterin (BH4) essential cofactor for tyrosine hydroxylase and nitric oxide synthase, on the 3,4-dihydroxyphenylalanine (L-DOPA) release from in vitro incubated striatal tissue. dl-6-methyl-5,6,7,8 tetrahydropterine (6-MPH4)-stimulated L-DOPA release in a concentration-dependent
M Bauer et al.
Journal of neurochemistry, 82(5), 1300-1310 (2002-10-03)
Glial cell line-derived neurotrophic factor (GDNF) protects dopaminergic neurones against toxic and physical damage. In addition, GDNF promotes differentiation and structural integrity of dopaminergic neurones. Here we show that GDNF can support the function of primary dopaminergic neurones by triggering
J R Bostwick et al.
Analytical biochemistry, 192(1), 125-130 (1991-01-01)
A radiometric assay for tyrosine hydroxylase employing a coupled nonenzymatic decarboxylation of L-[14C]Dopa formed from L-[14C]tyrosine has been adapted for performance in a 96 microwell culture plate. The method uses an easily manufactured plate holder to compress blotting paper impregnated
F García-Molina et al.
Biochimica et biophysica acta, 1794(12), 1766-1774 (2009-08-22)
There is controversy in the literature concerning the action of tetrahydropterines on the enzyme tyrosinase and on melanogenesis in general. In this study, we demonstrate that tetrahydropterines can inhibit melanogenesis in several ways: i) by non-enzymatic inhibition involving purely chemical
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica