M2147
Mevinolin from Aspergillus sp.
≥98% (HPLC)
Sinônimo(s):
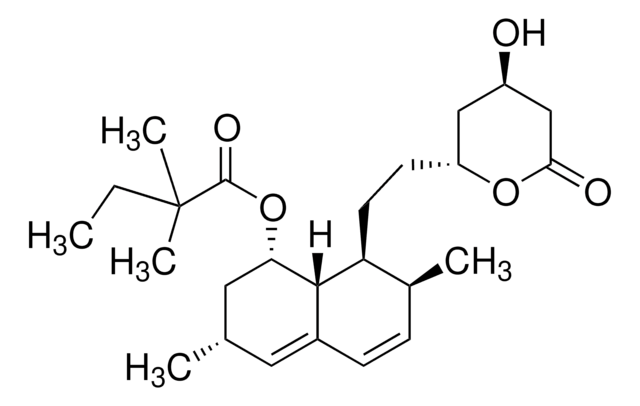
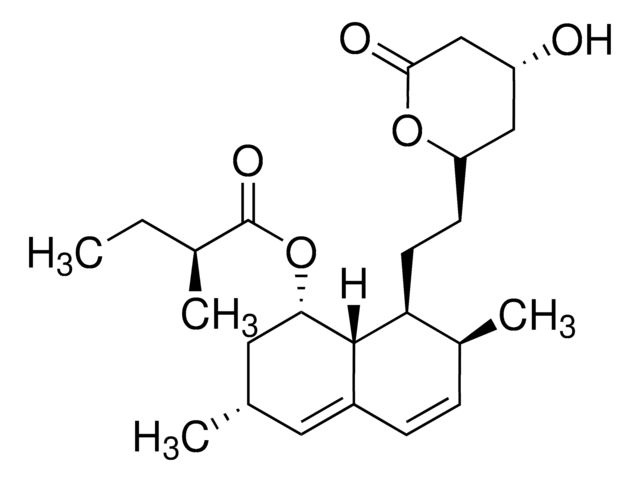
2-Methyl-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)ethyl]-1-naphthalenyl ester butanoic acid, 6-α-Methylcompactin, Lovastatin, Monacolin K
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥98% (HPLC)
Formulário
crystalline powder
pf
175.4 °C
solubilidade
ethanol: 9.80-10.20 mg/mL, clear, colorless to faintly yellow
originador
Abbott
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
[H][C@]12[C@H](C[C@@H](C)C=C1C=C[C@H](C)[C@@H]2CC[C@@H]3C[C@@H](O)CC(=O)O3)OC(=O)[C@@H](C)CC
InChI
1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1
chave InChI
PCZOHLXUXFIOCF-BXMDZJJMSA-N
Informações sobre genes
human ... HMGCR(3156)
rat ... Hmgcr(25675)
Procurando produtos similares? Visita Guia de comparação de produtos
Ações bioquímicas/fisiológicas
Características e benefícios
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Carc. 2 - Repr. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
The amount of cholesterol that is synthesized in the liver is tightly regulated by dietary cholesterol levels. LDL receptors regulate the cellular transport of lipid rich low density lipoprotein (LDL) particles.
Terpenes comprise the largest and most diverse class of secondary metabolites; approximately 55,000 compounds have been identified to date.
Randomized controlled clinical studies have suggested 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) are effective in both primary and secondary prevention of cardiovascular disease (CVD) events.
Conteúdo relacionado
Discover Bioactive Small Molecules for ADME/Tox
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica