M0534
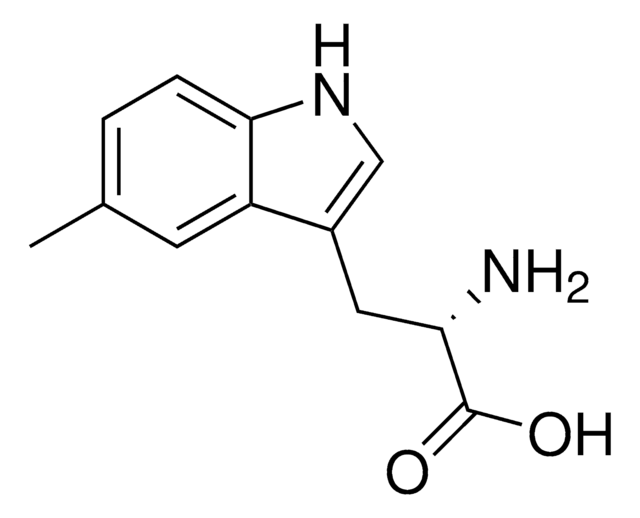
5-Methyl-DL-tryptophan
tryptophan analog
Sinônimo(s):
5-Methyltryptophan
Faça loginpara ver os preços organizacionais e de contrato
About This Item
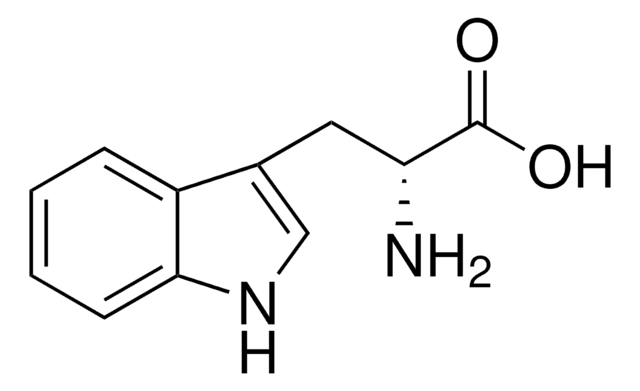
Fórmula empírica (Notação de Hill):
C12H14N2O2
Número CAS:
Peso molecular:
218.25
Beilstein:
20225
Número CE:
Número MDL:
Código UNSPSC:
12352209
ID de substância PubChem:
NACRES:
NA.26
Produtos recomendados
Nível de qualidade
Ensaio
≥97% (TLC)
Formulário
powder
cor
white to faint yellow
pf
280-282 °C
aplicação(ões)
peptide synthesis
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
Cc1ccc2[nH]cc(CC(N)C(O)=O)c2c1
InChI
1S/C12H14N2O2/c1-7-2-3-11-9(4-7)8(6-14-11)5-10(13)12(15)16/h2-4,6,10,14H,5,13H2,1H3,(H,15,16)
chave InChI
HUNCSWANZMJLPM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Amelioration of colitis through modulation of gut microbiota: The metabolite 5-Methyl-ᴅʟ-tryptophan (5-MT), derived from Angelica sinensis polysaccharides, was found to ameliorate colitis by modulating gut microbiota and the TLR4/MyD88/NF-κB signaling pathway. This suggests a potential application of 5-MT in inflammatory bowel disease research and therapy (Zou et al., 2023).
- Enzymatic synthesis of tryptophan derivatives: A study on the one-pot enantioselective synthesis of (S)-spirobrassinin and non-natural (S)-methylspirobrassinin from amino acids highlights a method using a turnip enzyme. This research outlines a novel approach to synthesizing tryptophan derivatives, potentially useful in biochemical assays (Ryu et al., 2021).
Ações bioquímicas/fisiológicas
5-Methyl-DL-tryptophan inhibits the synthesis of anthranilate compounds that are the first steps in the biosynthesis of tryptophan in Neurospora crassa. 5-Methyl-DL-tryptophan is a corepressor of the E. coli trp repressor.
5-Methyl-DL-tryptophan may be used to select genetic mutants of PS strain of Methanococcus voltae (archaebacteria). 5-Methyl-tryptophan is a repressor trp operon expression. 5-Methyl-tryptophan is a substrate for tryptophanase. 5-Methyl-tryptophan inhibits the induction of anthranilate synthase activity by elicitors in oats.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
X H Zhang et al.
Plant physiology, 127(1), 131-141 (2001-09-13)
Anthranilate synthase (AS), the control enzyme of the tryptophan (Trp) biosynthetic pathway, is encoded by nuclear genes, but is transported into the plastids. A tobacco (Nicotiana tabacum) cDNA (ASA2) encoding a feedback-insensitive tobacco AS alpha-subunit was transformed into two different
G Lester
Journal of bacteriology, 96(5), 1768-1773 (1968-11-01)
The in vivo regulation of intermediate reactions in the pathway of tryptophan synthesis in Neurospora crassa was examined in a double mutant (tr-2, tr-3) which lacks the functions of the first and last enzymes in the pathway from chorismic acid
Vered Tzin et al.
The New phytologist, 194(2), 430-439 (2012-02-03)
The shikimate pathway of plants mediates the conversion of primary carbon metabolites via chorismate into the three aromatic amino acids and to numerous secondary metabolites derived from them. However, the regulation of the shikimate pathway is still far from being
Tetsuya Matsukawa et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 57(1-2), 121-128 (2002-04-03)
Oat phytoalexins, avenanthramides, are a series of substituted hydroxycinnamic acid amides with anthranilate. The anthranilate in avenanthramides is biosynthesized by anthranilate synthase (AS, EC 4.1.3.27). Induction of anthranilate synthase activity was investigated in oat leaves treated with oligo-N-acetylchitooligosaccharide elicitors. AS
E I Hyde et al.
European journal of biochemistry, 201(3), 569-579 (1991-11-01)
The Escherichia coli trp repressor binds to the trp operator in the presence of tryptophan, thereby inhibiting tryptophan biosynthesis. Tryptophan analogues lacking the alpha-amino group act as inducers of trp operon expression. We have used one- and two-dimensional 1H-NMR spectroscopy
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica