L6250
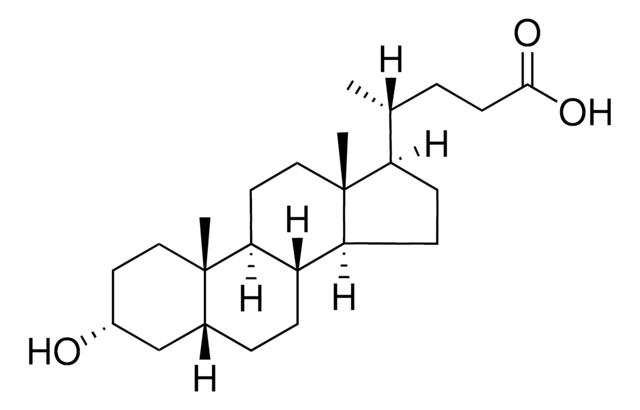
Lithocholic acid
≥95%
Sinônimo(s):
3α-Hydroxy-5β-cholan-24-oic acid, 3α-Hydroxy-5β-cholanic acid, 5β-Cholan-24-oic acid-3α-ol
About This Item
Produtos recomendados
fonte biológica
bovine bile
synthetic
Ensaio
≥95%
peso molecular
376.57 g/mol
pf
183-188 °C (lit.)
grupo funcional
carboxylic acid
Condições de expedição
ambient
temperatura de armazenamento
room temp
cadeia de caracteres SMILES
[H][C@]12CC[C@@]3([H])[C@]4([H])CC[C@H]([C@H](C)CCC(O)=O)[C@@]4(C)CC[C@]3([H])[C@@]1(C)CC[C@@H](O)C2
InChI
1S/C24H40O3/c1-15(4-9-22(26)27)19-7-8-20-18-6-5-16-14-17(25)10-12-23(16,2)21(18)11-13-24(19,20)3/h15-21,25H,4-14H2,1-3H3,(H,26,27)/t15-,16-,17-,18+,19-,20+,21+,23+,24-/m1/s1
chave InChI
SMEROWZSTRWXGI-HVATVPOCSA-N
Informações sobre genes
human ... POLA1(5422) , TOP2A(7153)
rat ... Polb(29240)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
This method is particularly useful in research into the role of individual bile acids as signaling molecules; suitable for clinical laboratories to investigate potential mechanisms linked to gut hormone profiles and glycemic control.
Conteúdo relacionado
Bile Acids (BA) are synthesized in the liver and play important roles in cholesterol homeostasis, absorption of vitamins and lipids, and various key metabolic processes.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica