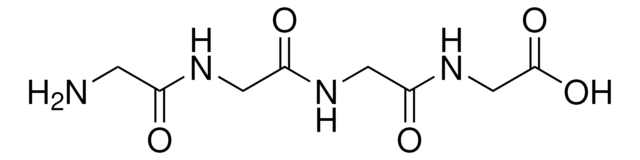
G5386
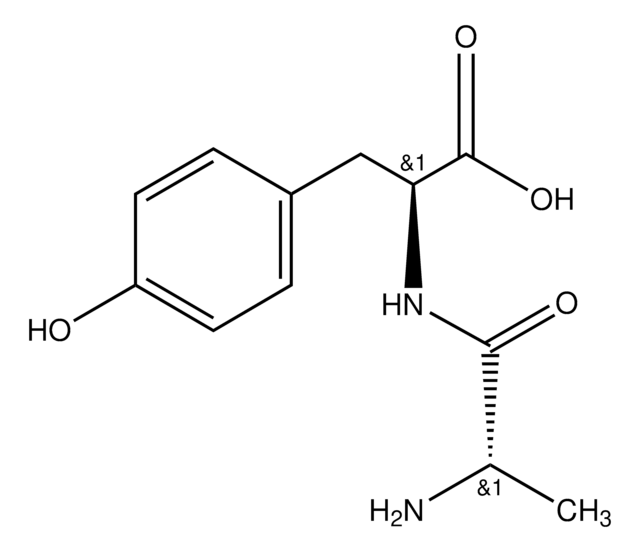
Gly-Gly-Tyr-Arg
powder, ≥97% (HPLC)
Sinônimo(s):
GGYR, Glycyl-glycyl-tyrosyl-arginine
About This Item
Produtos recomendados
product name
Gly-Gly-Tyr-Arg, ≥97% (HPLC)
fonte biológica
synthetic (organic)
Ensaio
≥97% (HPLC)
forma
powder
solubilidade
water: 2 mg/mL, clear, colorless
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
NCC(=O)NCC(=O)NC(Cc1ccc(O)cc1)C(=O)NC(CCCNC(N)=N)C(O)=O
InChI
1S/C19H29N7O6/c20-9-15(28)24-10-16(29)25-14(8-11-3-5-12(27)6-4-11)17(30)26-13(18(31)32)2-1-7-23-19(21)22/h3-6,13-14,27H,1-2,7-10,20H2,(H,24,28)(H,25,29)(H,26,30)(H,31,32)(H4,21,22,23)
chave InChI
FJPHHBGPPJXISY-UHFFFAOYSA-N
Aplicação
- in the synthesis of the dimethoxyphosphotyrosine adduct to determine if diethoxyphosphate tyrosine (depY), a monoclonal antibody could distinguish between dimethoxyphosphotyrosine and diethoxyphosphotyrosine
- to graft on to a soft polyacrylamide for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) to perform high-resolution separation of peptides
- as a model peptide to react with 3,3-dithiobis(sulfosuccinimidyl propionate) (DTSSP) to understand the chemistry of DTSSP and similar cross-linking reagents
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Papain is a cysteine protease of the peptidase C1 family. Papain consists of a single polypeptide chain with three disulfide bridges and a sulfhydryl group necessary for activity of the enzyme.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica