D7696
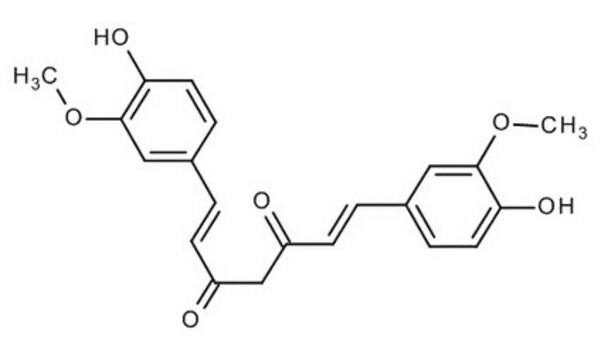
Demethoxycurcumin
≥98% (HPLC), powder, LPS-induced nitric oxide (NO) production inhibitor
Sinônimo(s):
(E,E)-1-(4-Hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione, Curcumin II, Desmethoxycurcumin, Monodemethoxycurcumin
About This Item
Produtos recomendados
Nome do produto
Demethoxycurcumin, ≥98% (HPLC)
Nível de qualidade
Ensaio
≥98% (HPLC)
Formulário
powder
condição de armazenamento
protect from light
cor
orange
solubilidade
DMSO: ≥10 mg/mL
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
COc1cc(\C=C\C(=O)CC(=O)\C=C\c2ccc(O)cc2)ccc1O
InChI
1S/C20H18O5/c1-25-20-12-15(6-11-19(20)24)5-10-18(23)13-17(22)9-4-14-2-7-16(21)8-3-14/h2-12,21,24H,13H2,1H3/b9-4+,10-5+
chave InChI
HJTVQHVGMGKONQ-LUZURFALSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Ações bioquímicas/fisiológicas
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica