C6019
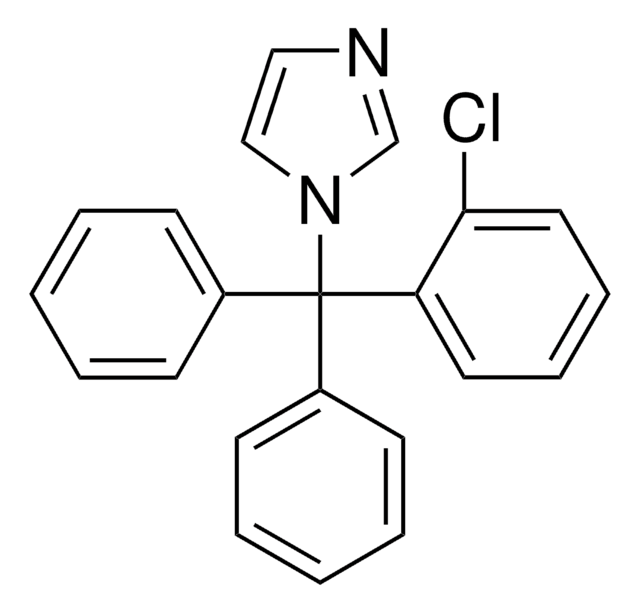
Clotrimazole
98.5-100.5% (dry basis), powder, Ca²⁺-activated K⁺ channels inhibitor
Sinônimo(s):
1-(o-Chloro-α,α-diphenylbenzyl)imidazole, 1-(o-Chlorotrityl)imidazole, 1-[(2-Chlorophenyl)diphenylmethyl]-1H-imidazole
About This Item
Produtos recomendados
Nome do produto
Clotrimazole,
Formulário
powder
espectro de atividade do antibiótico
fungi
Modo de ação
cell membrane | interferes
protein synthesis | interferes
originador
Schering Plough
cadeia de caracteres SMILES
Clc1ccccc1C(c2ccccc2)(c3ccccc3)n4ccnc4
InChI
1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H
chave InChI
VNFPBHJOKIVQEB-UHFFFAOYSA-N
Informações sobre genes
human ... ABCB1(5243) , CYP17A1(1586) , CYP3A4(1576)
mouse ... Abcb1a(18671) , Abcb1b(18669)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- to study the upregulation of the gene ERG11 that codes for an azole target enzyme lanosterol demethylase, in Candida species, upon treatment with azole antibiotics
- to study the development of resistance in Candida species isolated from patients undergoing prolonged antifungal treatment
- to induce stress granules via mitochondrial stress
- for the inhibition of in vitro formation of high density sickle cells induced by treatment with 1-chloro-2,4-dinitrobenzene (CDNB)
- to inhibit cytochrome P450 enzyme in cell cultures
Ações bioquímicas/fisiológicas
Características e benefícios
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Irrit. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Equipamento de proteção individual
dust mask type N95 (US), Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica