C0737
Cilostazol
≥98% (HPLC), powder
Sinônimo(s):
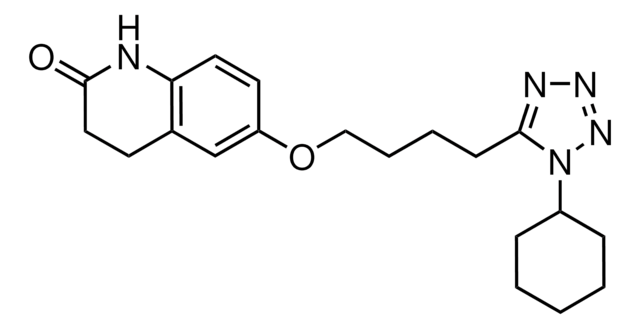
6-[4-(1-Cyclohexyl-1H-tetrazol-5-yl)-butoxy]-3,4-dihydro-2(1H)-quinolinone, OPC 13013, OPC 21, Pletaal
About This Item
Produtos recomendados
Ensaio
≥98% (HPLC)
Formulário
powder
cor
off-white
solubilidade
DMSO: 10 mg/mL, clear
originador
Otsuka Pharma
cadeia de caracteres SMILES
O=C1CCc2cc(OCCCCc3nnnn3C4CCCCC4)ccc2N1
InChI
1S/C20H27N5O2/c26-20-12-9-15-14-17(10-11-18(15)21-20)27-13-5-4-8-19-22-23-24-25(19)16-6-2-1-3-7-16/h10-11,14,16H,1-9,12-13H2,(H,21,26)
chave InChI
RRGUKTPIGVIEKM-UHFFFAOYSA-N
Informações sobre genes
human ... PDE3A(5139) , PDE3B(5140)
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
- to reduce Madin–Darby cell line (MDCK) proliferation through c-Myc down-regulation
- in the in vitro assessment of toxin delivery in T84 intestinal epithelial cells
- to induce adenosine triphosphate (ATP) release in white adipocytes
Ações bioquímicas/fisiológicas
Características e benefícios
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Repr. 2
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Conteúdo relacionado
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica