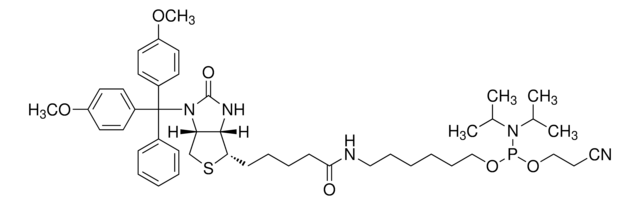
AL11430
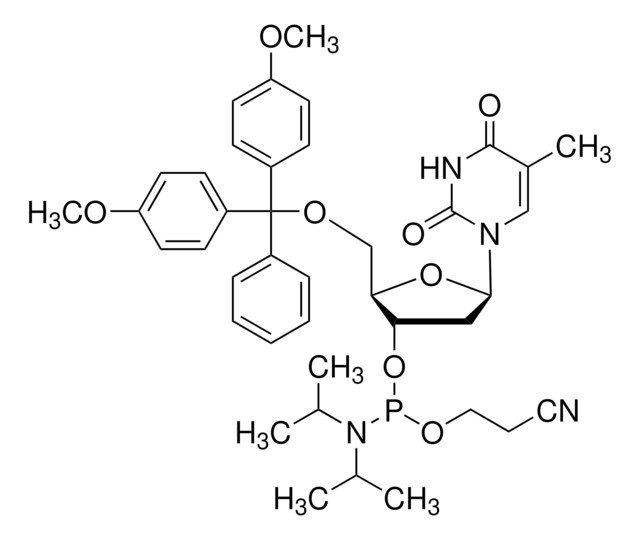
DMT-locA(bz) Phosphoramidite
configured for ABI
About This Item
Produtos recomendados
fonte biológica
non-animal source (BSE/TSE no Risk)
Nível de qualidade
linha de produto
Proligo Reagents
Ensaio
≥98.0% (31P-NMR)
≥98.0% (reversed phase HPLC)
forma
powder
Impurezas
≤3 wt. % Residual Solvent Content
<0.4% Water Content (Karl Fischer)
<0.5% Single unspecified Impurity (reversed phase HPLC)
cor
white to off-white
solubilidade
soluble, clear, colorless
absorção
<0.1 in acetonitrile at 0.2 M
adequação
conforms to structure for H-NMR
conforms to structure for LC-MS
compatibilidade
configured for ABI
temperatura de armazenamento
−20°C
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
With the exception of the LNA monomers, LNA synthesis is accomplished with the same reagents as DNA synthesis. LNA phosphoramidites from Merck are diluted with dry acetonitrile, except for locMeC(bz)-phosphoramidite. This phosphoramidite requires the application of a cosolvent to prevent crystallization from the solution on the synthesizer. Dichloromethane or tetrahydrofuran (THF) can be applied as co-solvents with acetonitrile to completely dissolve locMeC(bz)-phosphoramidite.
Características e benefícios
- LNA oligonucleotides are prepared by phosphoramidite chemistry
- Standard DNA synthesizer platforms can be employed. No change is required in the reagents commonly used for DNA synthesis
- To further enhance the hybridization characteristics of LNA, 5-methyl-cytidine is employed instead of cytidine
- LNA monomers are as soluble in acetonitrile as their DNA counterparts (except for the 5-methyl-cytidine derivative, which requires the application of 10-20%, dichloromethane as a co-solvent)
- Mixmer oligonucleotides containing LNA, DNA and/or RNA monomers can be assembled easily
- LNA oligonucleotides with predefined melting temperatures (Tm) can be designed and prepared
Outras notas
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Certificados de análise (COA)
Busque Certificados de análise (COA) digitando o Número do Lote do produto. Os números de lote e remessa podem ser encontrados no rótulo de um produto após a palavra “Lot” ou “Batch”.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica