A7236
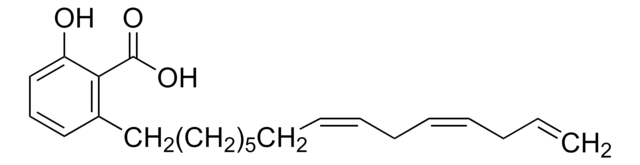
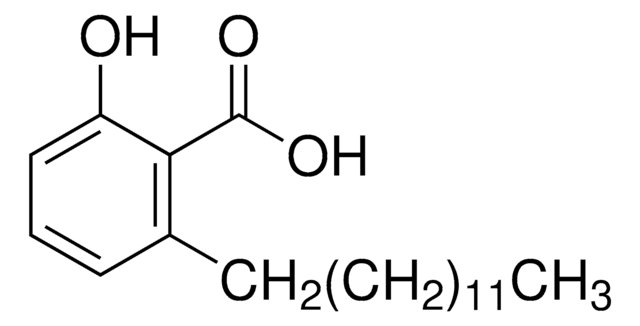
Anacardic acid
Sinônimo(s):
2-Hydroxy-6-pentadecylbenzoic acid, 22:0-Anacardic acid, 6-Pentadecylsalicylic acid
About This Item
Produtos recomendados
Formulário
powder
Nível de qualidade
condição de armazenamento
protect from light
cor
white to beige
solubilidade
DMSO: ≥20 mg/mL
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
CCCCCCCCCCCCCCCc1cccc(O)c1C(O)=O
InChI
1S/C22H36O3/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-16-19-17-15-18-20(23)21(19)22(24)25/h15,17-18,23H,2-14,16H2,1H3,(H,24,25)
chave InChI
ADFWQBGTDJIESE-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- as a histone acetylase (HAT) inhibitor to study its effects on rat cortical neurons
- as a positive control in acetylation assay in vitro
- as an acetylase inhibitor to study its effects on the ribonucleic acid export 1 (Rae-1) protein acetylation that was transfected in human embryonic kidney cells
Ações bioquímicas/fisiológicas
Características e benefícios
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Conteúdo relacionado
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica