90081
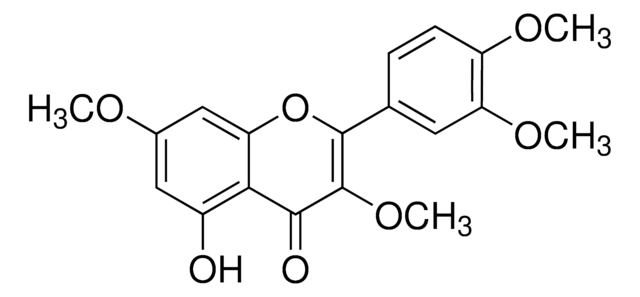
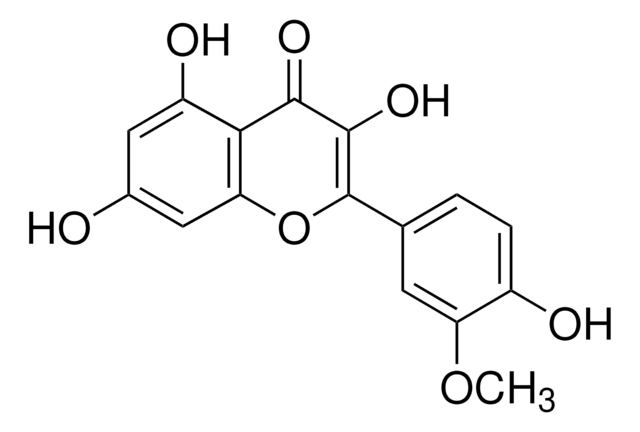
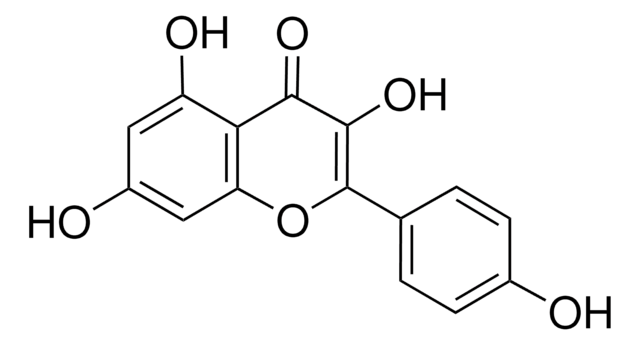
3-O-Methylquercetin
≥97% (HPLC)
Sinônimo(s):
5,7,3′ ,4′ -Tetrahydroxy-3-methoxyflavone, Quercetin 3-O-methyl ether
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C16H12O7
Número CAS:
Peso molecular:
316.26
Beilstein:
324509
Número MDL:
Código UNSPSC:
12352202
ID de substância PubChem:
NACRES:
NA.32
Produtos recomendados
Ensaio
≥97% (HPLC)
Formulário
powder
cadeia de caracteres SMILES
COC1=C(Oc2cc(O)cc(O)c2C1=O)c3ccc(O)c(O)c3
InChI
1S/C16H12O7/c1-22-16-14(21)13-11(20)5-8(17)6-12(13)23-15(16)7-2-3-9(18)10(19)4-7/h2-6,17-20H,1H3
chave InChI
WEPBGSIAWZTEJR-UHFFFAOYSA-N
Ações bioquímicas/fisiológicas
3-O-Methylquercetin significantly inhibits cyclic adenosine monophosphate- (cAMP-) and cyclic guanosine monophosphate- (cGMP-) phosphodiesterase activity. It possess anti-inflammatory, bronchodilating properties and used in treatment of asthma. It suppresses the total inflammatory cells, tumor necrosis factor-α (TNF-α) and attenuates the production of interleukins.
3-O-Methylquercetin is a metabolite in flavone and flavonol biosynthesis. It is a naturally occurring compound present in various plants, and was shown to have potent anticancer-promoting, antioxidant, antiallergy, and antimicrobial activity, and showed strong anti-viral activity inhibition of tomato ringspot virus.
Embalagem
Bottomless glass bottle. Contents are inside inserted fused cone.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 3 Oral
Código de classe de armazenamento
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
3-O-methylquercetin more selectively inhibits phosphodiesterase subtype 3
Ko WC, et al.
Planta Medica, 69(04), 310-315 (2003)
Quercetin-3-methyl ether suppresses proliferation of mouse epidermal JB6 P+ cells by targeting ERKs.
Jixia Li et al.
Carcinogenesis, 33(2), 459-465 (2011-12-06)
Chemoprevention has been acknowledged as an important and practical strategy for the management of skin cancer. Quercetin-3-methyl ether, a naturally occurring compound present in various plants, has potent anticancer-promoting activity. We identified this compound by in silico virtual screening of
Ana Paula Preczenhak et al.
Food chemistry, 286, 600-607 (2019-03-05)
This study investigated the effectiveness of cysteine in conservation of bioactive compounds and the antioxidant capacity of minimally processed red beet. After red beet minimal processing increasing cysteine concentrations were applied, corresponding to control, 2 mM, 4 mM, 8 mM and 16 mM. Assay
C Angeloni et al.
Biochimie, 89(1), 73-82 (2006-10-19)
The aim of this study was to investigate the potential of quercetin and two of its "in vivo" metabolites, 3'-O-methyl quercetin and 4'-O-methyl quercetin, to protect H9c2 cardiomyoblasts against H(2)O(2)-induced oxidative stress. As limited data are available regarding the potential
Clement K Ameho et al.
The Journal of nutritional biochemistry, 19(7), 467-474 (2007-10-02)
Dietary antioxidants interact in a dynamic fashion, including recycling and sparing one another, to decrease oxidative stress. Limited information is available regarding the interrelationships in vivo between quercetin and vitamin E. We investigated the antioxidant activity and metabolism of quercetin
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica