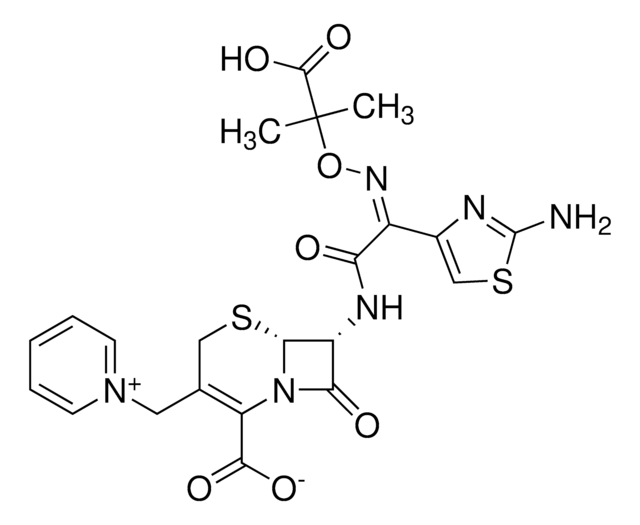
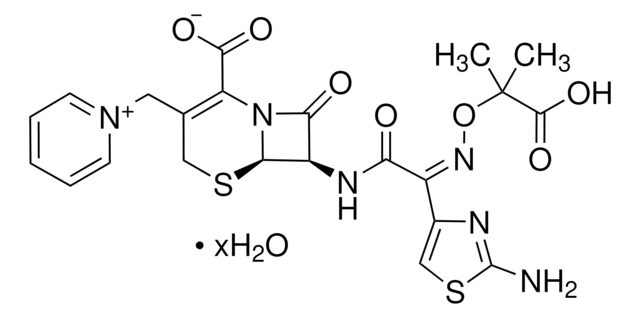
Y0000420
Cefotaxime acid
European Pharmacopoeia (EP) Reference Standard
Sinônimo(s):
Cefotaxime
About This Item
Produtos recomendados
grau
pharmaceutical primary standard
família API
cefotaxime
fabricante/nome comercial
EDQM
aplicação(ões)
pharmaceutical (small molecule)
Formato
neat
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
[Na+].[s]1c(nc(c1)\C(=N\OC)\C(=N\[C@@H]2[C@@H]3SCC(=C(N3C2=O)C(=O)O)COC(=O)C)\[O-])N
InChI
1S/C16H17N5O7S2.Na/c1-6(22)28-3-7-4-29-14-10(13(24)21(14)11(7)15(25)26)19-12(23)9(20-27-2)8-5-30-16(17)18-8;/h5,10,14H,3-4H2,1-2H3,(H2,17,18)(H,19,23)(H,25,26);/q;+1/p-1/b20-9-;/t10-,14-;/m0./s1
chave InChI
AZZMGZXNTDTSME-XJTDMATHSA-M
Descrição geral
Aplicação
Embalagem
Outras notas
produto relacionado
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica