Y0000109
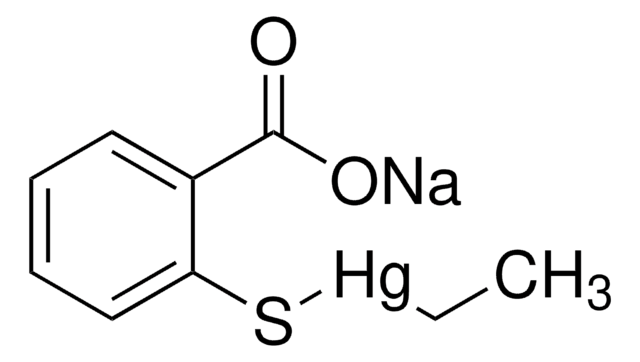
Timerosal
European Pharmacopoeia (EP) Reference Standard
Sinônimo(s):
Etilmercuritiossalicilato de sódio, Mercúrio-([o-carboxifenil]tio)etil, Ácido 2-(etilmercuriomercapto)benzoico, Ácido etilmercuritiossalicílico
About This Item
Produtos recomendados
grau
pharmaceutical primary standard
família API
thimerosal
fabricante/nome comercial
EDQM
pf
234-237 °C (dec.) (lit.)
aplicação(ões)
pharmaceutical (small molecule)
formato
neat
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
[Na+].CC[Hg]Sc1ccccc1C([O-])=O
InChI
1S/C7H6O2S.C2H5.Hg.Na/c8-7(9)5-3-1-2-4-6(5)10;1-2;;/h1-4,10H,(H,8,9);1H2,2H3;;/q;;2*+1/p-2
chave InChI
RTKIYNMVFMVABJ-UHFFFAOYSA-L
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
For further information and support please go to the website of the issuing Pharmacopoeia.
Aplicação
Ações bioquímicas/fisiológicas
Embalagem
Outras notas
Exoneração de responsabilidade
The product is not intended for use as a biocide under global biocide regulations, including but not limited to US EPA′s Federal Insecticide Fungicide and Rodenticide Act , European Biocidal Products Regulation, Canada′s Pest Management Regulatory Agency, Turkey′s Biocidal Products Regulation, Korea′s Consumer Chemical Products and Biocide Safety Management Act (K-BPR) and others.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 1 Dermal - Acute Tox. 2 Inhalation - Acute Tox. 2 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - STOT RE 2 Oral
Órgãos-alvo
Kidney
Código de classe de armazenamento
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica