PHR1363
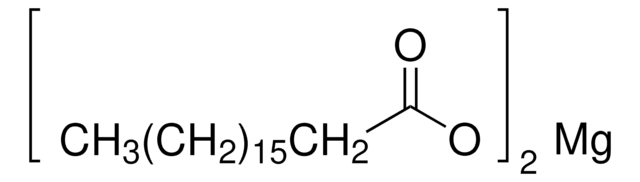
Magnesium Stearate
Pharmaceutical Secondary Standard; Certified Reference Material
Sinônimo(s):
Magnesium stearate excipient standard
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Produtos recomendados
grau
certified reference material
pharmaceutical secondary standard
Nível de qualidade
família API
magnesium stearate
embalagem
ampule of 2.5 g
aplicação(ões)
pharmaceutical (small molecule)
formato
neat
temperatura de armazenamento
2-30°C
Descrição geral
Magnesium stearate is commonly utilized as a lubricant in pharmaceutical formulations to avoid the excipient blend from the formulation contents from sticking to the manufacturing equipment.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Aplicação
Magnesium stearate may be used as an excipient for the manufacturing of atorvastatin drug and poloxamers. The determination of the analyte in pharmaceutical formulations can be carried out by chromatography and colorimetric methods.
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
Nota de análise
These secondary standards offer multi-traceability to the USP, EP (PhEur) and BP primary standards, where they are available.
Outras notas
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Nota de rodapé
To see an example of a Certificate of Analysis for this material enter LRAA1467 in the slot below. This is an example certificate only and may not be the lot that you receive.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
nwg
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Analysis of magnesium from magnesium stearate in pharmaceutical tablet formulations using hydrophilic interaction liquid chromatography with nano quantity analyte detection
Risley DS, et al.
Journal of Pharmaceutical and Biomedical Analysis, 78, 112-117 (2013)
Liquid chromatographic determination of atorvastatin in bulk drug, tablets, and human plasma
Altuntas TG and Erk N
Journal of Liquid Chromatography and Related Technologies, 27(1), 83-93 (2004)
Quantitation of poloxamers in pharmaceutical formulations using size exclusion chromatography and colorimetric methods
Mao Y, et al.
Journal of Pharmaceutical and Biomedical Analysis, 35(5), 1127-1142 (2004)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica