K1637
Canamicina
meets USP testing specifications, powder
Sinônimo(s):
Canamicina, Canamicina A
About This Item
Produtos recomendados
fonte biológica
Streptomyces kanamyceticus
Nível de qualidade
Agency
USP/NF
meets USP testing specifications
Formulário
powder
potência
≥750 μg per mg
solubilidade
H2O: 10-50 mg/mL (As a stock solution. Stock solutions should be stored at 2-8°C. Stable at 37°C for 5 days.)
espectro de atividade do antibiótico
Gram-negative bacteria
Gram-positive bacteria
mycobacteria
mycoplasma
aplicação(ões)
pharmaceutical (small molecule)
Modo de ação
protein synthesis | interferes
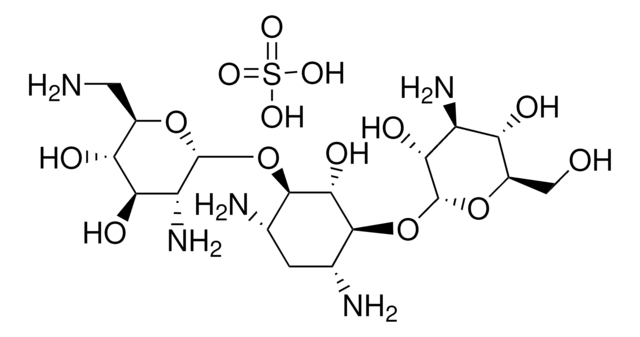
cadeia de caracteres SMILES
OS(O)(=O)=O.NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C18H36N4O11.H2O4S/c19-2-6-10(25)12(27)13(28)18(30-6)33-16-5(21)1-4(20)15(14(16)29)32-17-11(26)8(22)9(24)7(3-23)31-17;1-5(2,3)4/h4-18,23-29H,1-3,19-22H2;(H2,1,2,3,4)/t4-,5+,6-,7-,8+,9-,10-,11-,12+,13-,14-,15+,16-,17-,18-;/m1./s1
chave InChI
OOYGSFOGFJDDHP-KMCOLRRFSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Descrição geral
Aplicação
Ações bioquímicas/fisiológicas
Modo de resistência: as enzimas modificadoras de aminoglicosídeo (incluindo acetiltransferase, fosfotransferase, nucleotidiltransferase) podem alterar esse antibiótico, prevenindo sua interação com os ribossomos.
Espectro da ação antimicrobiana: O sulfato de canamicina é eficaz contra bactérias gram negativas e gram positivas e micoplasma.
Características e benefícios
- High quality antibiotic suitable for mulitple research applications
- meets USP testing specifications
Nota de preparo
Solutions are stable at 37°C for approximately 5 days. Aqueous stock solutions can be stored at 2-8°C for long term storage.
Armazenamento e estabilidade
Outras notas
produto comparável
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Repr. 1B
Código de classe de armazenamento
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
Classe de risco de água (WGK)
WGK 2
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica