90450
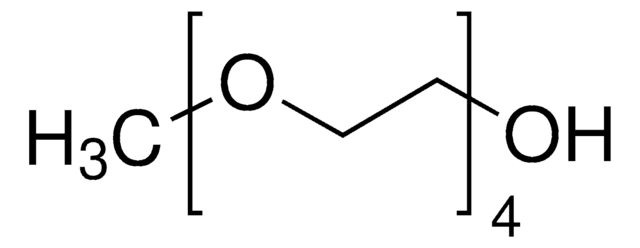
Triethylene glycol monomethyl ether
purum, ≥97.0% (GC)
Sinônimo(s):
Methyltriglycol
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula linear:
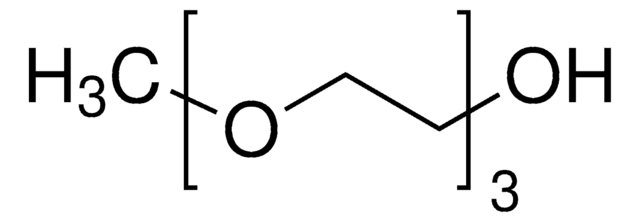
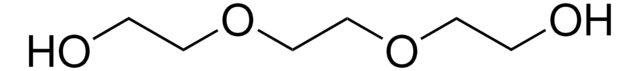
CH3(OCH2CH2)3OH
Número CAS:
Peso molecular:
164.20
Beilstein:
1700198
Número CE:
Número MDL:
Código UNSPSC:
12162002
ID de substância PubChem:
NACRES:
NA.23
Produtos recomendados
densidade de vapor
5.66 (vs air)
Nível de qualidade
pressão de vapor
<0.01 mmHg ( 20 °C)
descrição
non-ionic
grau
purum
Ensaio
≥97.0% (GC)
Formulário
liquid
peso molecular
164.20 g/mol
índice de refração
n20/D 1.439 (lit.)
n20/D 1.439
p.e.
122 °C/10 mmHg (lit.)
densidade
1.026 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
COCCOCCOCCO
InChI
1S/C7H16O4/c1-9-4-5-11-7-6-10-3-2-8/h8H,2-7H2,1H3
chave InChI
JLGLQAWTXXGVEM-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Triethylene glycol monomethyl ether (TEGME) was used in the following studies:
- To study the structural and thermosensitive properties of phosphazene derivatives having glycol and amino acids groups.
- Synthesis of amphiphilic o-phenylene ethylene oligomers.
- Preparation of pegylated zinc(II) phthalocyanines.
- Modification of 2-OH positions in cyclodextrin thioethers, which are used for transport of hydrophobic drugs.
- Functionalizing polymethacrylic acid (PMMA), hence increasing inhibition in crack generation in TEGMA-PMMA compared to unmodified PMMA electrodes.
- Preparation of difunctionalized ionic liquids (ILs).
- As terminal groups for cationic dendrons, which was further used to encapsulate an inorganic polyanionic cluster.
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 1
Ponto de fulgor (°F)
230.0 °F - closed cup
Ponto de fulgor (°C)
110 °C - closed cup
Equipamento de proteção individual
Eyeshields, Gloves
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Lisa F Becker et al.
Beilstein journal of organic chemistry, 10, 2920-2927 (2015-01-01)
Methyl and ethyl thioether groups were introduced at all primary positions of α-, β-, and γ-cyclodextrin by nucleophilic displacement reactions starting from the corresponding per-(6-deoxy-6-bromo)cyclodextrins. Further modification of all 2-OH positions by etherification with iodo terminated triethylene glycol monomethyl ether
Synthesis and Characterization of amphiphilic -co-Phenylene ethynylene oligomers
<B>Slutsky MM, et al.</B>
New. J. Chem., 32, 670-675 (2008)
Sandra D Hojniak et al.
The journal of physical chemistry. B, 118(26), 7440-7449 (2014-06-05)
Novel difunctionalized ionic liquids (ILs) containing a triethylene glycol monomethyl ether chain and a nitrile group on a pyrrolidinium or imidazolium cation have been synthesized and incorporated into supported ionic liquid membranes (SILMs). These ILs exhibit ca. 2.3 times higher
A Systematic Investigation of Polymer Binder Flexibility on the Electrode Performance of Lithium-Ion Batteries
Yuca N, et al.
ACS Applied Materials & Interfaces, 6(19), 17111-17118 (2014)
Hailong Chen et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(33), 11051-11061 (2013-07-03)
A series of cationic dendrons bearing triethylene glycol monomethyl ether terminal groups of different generations have been synthesized and used to encapsulate an inorganic polyanionic cluster [K(12.5)Na(1.5)(NaP5W30O110)] through electrostatic interactions. The resulting dendritic cation-encapsulated polyoxometalate (POM) complexes, cluster-dendrimers, are soluble
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica