69484
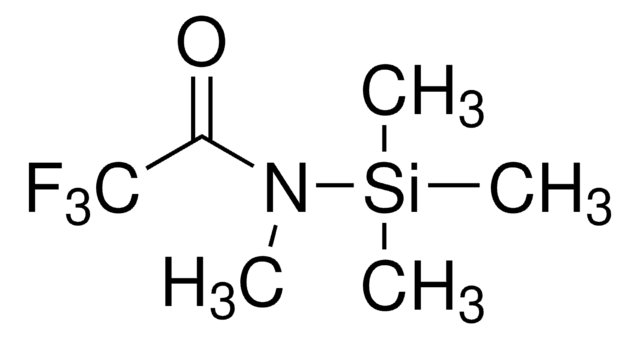
N-Methyl-N-trimethylsilylheptafluorobutyramide
for GC derivatization, LiChropur™, ≥90% (GC)
Sinônimo(s):
N-Trimethylsilyl-N-methylheptafluorobutyramide, MSHFBA
About This Item
Produtos recomendados
grau
for GC derivatization
Nível de qualidade
Ensaio
≥90% (GC)
forma
liquid
qualidade
LiChropur™
adequação da reação
reagent type: derivatization reagent
reaction type: Silylations
técnica(s)
gas chromatography (GC): suitable
índice de refração
n20/D 1.353 (lit.)
n20/D 1.353
pb
148 °C (lit.)
densidade
1.254 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
CN(C(=O)C(F)(F)C(F)(F)C(F)(F)F)[Si](C)(C)C
InChI
1S/C8H12F7NOSi/c1-16(18(2,3)4)5(17)6(9,10)7(11,12)8(13,14)15/h1-4H3
chave InChI
CMXKINNDZCNCEI-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
- Simultaneous quantitation of cocaine, opiates, and their metabolites in human hair by positive ion chemical ionization gas chromatography-mass spectrometry: This study demonstrates the application of N-Methyl-N-trimethylsilylheptafluorobutyramide in forensic toxicology to analyze drug residues in human hair, providing a robust method for detecting such compounds at trace levels (Höld KM, Wilkins DG, Rollins DE, Joseph RE Jr, Cone EJ, 1998).
- Detection of stanozolol in hair by negative ion chemical ionization mass spectrometry: The research utilizes N-Methyl-N-trimethylsilylheptafluorobutyramide for the sensitive detection of stanozolol, a performance-enhancing steroid, in hair samples. This method is particularly useful in sports doping analyses to ensure fair play (Höld KM, Wilkins DG, Crouch DJ, Rollins DE, Maes RA, 1996).
Outras notas
Informações legais
produto relacionado
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
91.4 °F - closed cup
Ponto de fulgor (°C)
33 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Results of a study involving the ability few Fluka silylating reagents to form GC-MS-compatible trimethylsilylmethyl derivatives of NSAIDs
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica