About This Item
Produtos recomendados
grau
analytical standard
Nível de qualidade
densidade de vapor
2.97 (vs air)
pressão de vapor
135 mmHg ( 17 °C)
Ensaio
≥99.5% (GC)
temperatura de autoignição
532 °F
prazo de validade
limited shelf life, expiry date on the label
Lim. expl.
~7.7 %
técnica(s)
HPLC: suitable
gas chromatography (GC): suitable
índice de refração
n20/D 1.376 (lit.)
n20/D 1.376
p.e.
64 °C (lit.)
densidade
0.664 g/mL at 25 °C (lit.)
aplicação(ões)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
Formato
neat
cadeia de caracteres SMILES
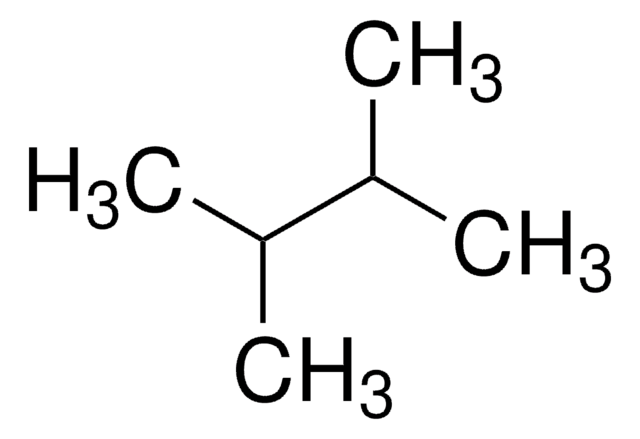
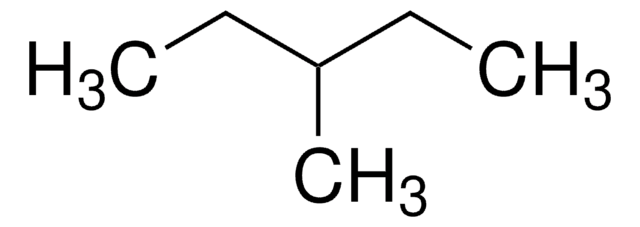
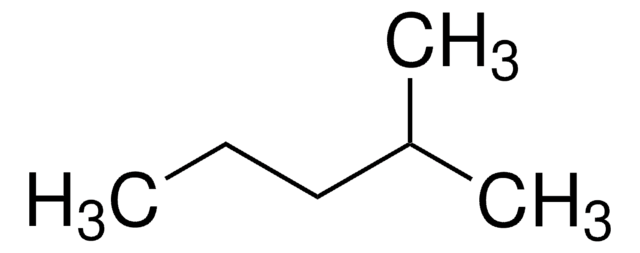
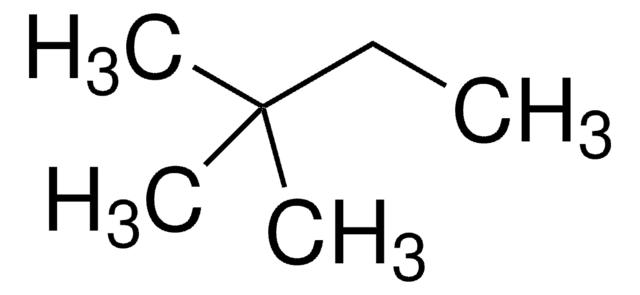
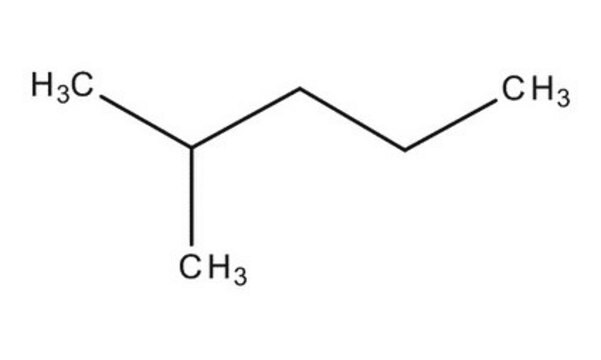
CCC(C)CC
InChI
1S/C6H14/c1-4-6(3)5-2/h6H,4-5H2,1-3H3
chave InChI
PFEOZHBOMNWTJB-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Aplicação
Produtos recomendados
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Central nervous system
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
19.4 °F - closed cup
Ponto de fulgor (°C)
-7 °C - closed cup
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Protocolos
Separation of 4-Methyl-2-pentanone; Dimethyl disulfide; Hexanal; 3-Methylpentane; Acetone
Protocol for GC Analysis of Hydrocarbons in Gasoline on Petrocol® DH
-1,3-Dimethylcyclopentane; 1,1-Dimethylcyclopentane; 2,2,3-Trimethylpentane; 2,2-Dimethylbutane; 2,2-Dimethylhexane; 2,2-Dimethylpentane; 2,3-Dimethylbutane; 2,3-Dimethylhexane; 2,4-Dimethylheptane; 2,4-Dimethylpentane; 2,5-Dimethylheptane; 2-Methylhexane; 2-Methylpentane; 3,3-Dimethylpentane; 3,4-Dimethylhexane; 3-Ethylpentane; 3-Methyloctane; 4-Methylheptane; Ethylbenzene; Ethylcyclopentane; 2,6-Dimethylheptane; 3-Ethylheptane
-Xylene; Nonane; Propylbenzene; Mesitylene; 1,2,4-Trimethylbenzene; 1,2,3-Trimethylbenzene; 1,3-Diethylbenzene; 1,4-Dimethyl-2-ethylbenzene; 1,2-Dimethyl-4-ethylbenzene; Durene; 1,2,3,5-Tetramethylbenzene; 1,2,3,5-Tetramethylbenzene; 2-Methylnaphthalene (β)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica