E-068
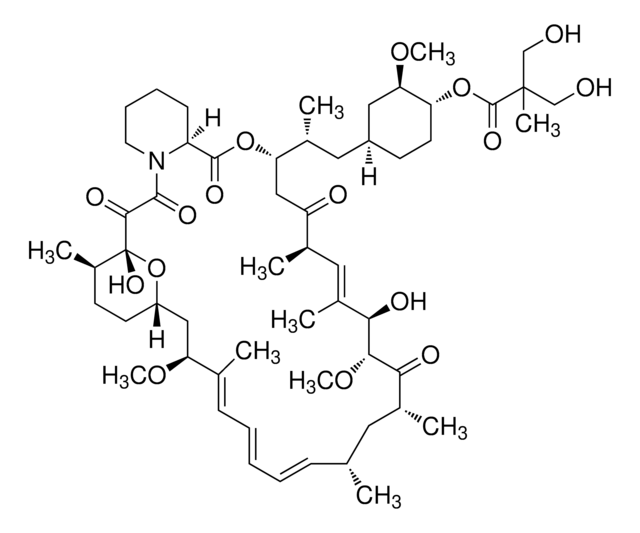
Everolimus solution
1.0 mg/mL in acetonitrile, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Produtos recomendados
grau
certified reference material
Nível de qualidade
Formulário
liquid
Características
(Snap-N-Spike®)
embalagem
ampule of 1 mL
fabricante/nome comercial
Cerilliant®
concentração
1.0 mg/mL in acetonitrile
técnica(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
aplicação(ões)
clinical testing
Formato
single component solution
Condições de expedição
dry ice
temperatura de armazenamento
−70°C
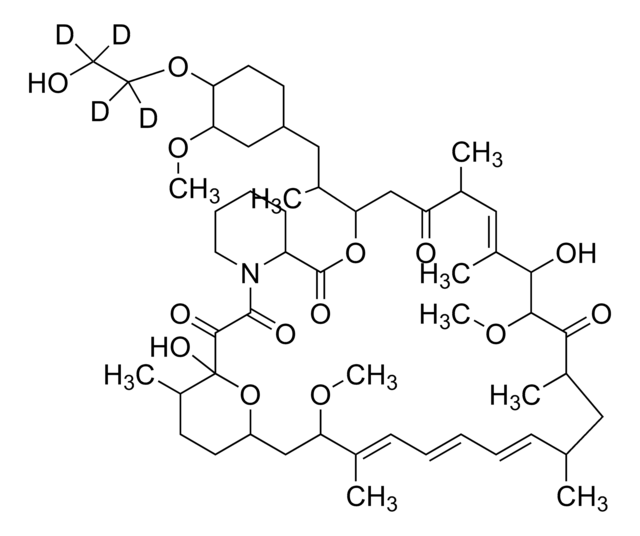
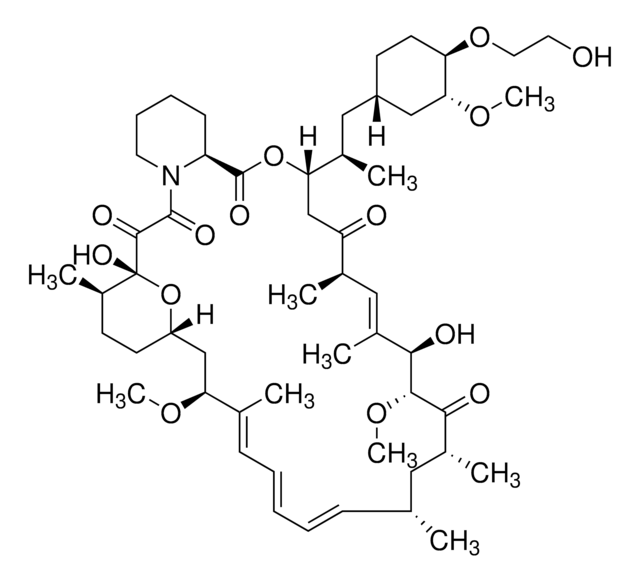
InChI
1S/C53H83NO14/c1-32-16-12-11-13-17-33(2)44(63-8)30-40-21-19-38(7)53(62,68-40)50(59)51(60)54-23-15-14-18-41(54)52(61)67-45(35(4)28-39-20-22-43(66-25-24-55)46(29-39)64-9)31-42(56)34(3)27-37(6)48(58)49(65-10)47(57)36(5)26-32/h11-13,16-17,27,32,34-36,38-41,43-46,48-49,55,58,62H,14-15,18-26,28-31H2,1-10H3/b13-11+,16-12+,33-17+,37-27+/t32-,34-,35-,36-,38-,39+,40+,41+,43-,44+,45+,46-,48-,49+,53-/m1/s1
chave InChI
HKVAMNSJSFKALM-GKUWKFKPSA-N
Informações sobre genes
human ... FKBP1A(2280)
Categorias relacionadas
Descrição geral
Aplicação
- Everolimus as a therapy for hepatoblastoma: Research demonstrates that Everolimus can induce autophagy-dependent ferroptosis in hepatoblastoma cells, highlighting its potential as a therapeutic agent in oncology research. This study provides insight into the mechanisms by which Everolimus can be utilized to target cancer cells through cell death pathways (Huang et al., 2024).
- Understanding oral mucosal injuries from mTOR inhibitors: A new hypothesis posits that oral mucosal injuries associated with mTOR inhibitors like Everolimus result from disruptions in cellular stress and apoptotic pathways. This study underscores the importance of understanding side effects in the context of targeted therapy for conditions such as cancers and immunosuppression (Sonis and Villa, 2023).
- Micellar formulation of Everolimus for neurological disorders: A stable micellar formulation of Everolimus (RAD001) has been developed for intracerebroventricular delivery, aimed at treating Alzheimer′s Disease and other neurological disorders. This formulation allows for direct brain administration, potentially enhancing the drug′s efficacy and safety profile (Gianessi et al., 2023).
- Pharmacokinetics in epilepsy treatment: The population pharmacokinetics of Everolimus were studied in patients with seizures associated with focal cortical dysplasia. This research aids in understanding the drug′s behavior in a specific neurological context, providing a foundation for dosing adjustments and therapeutic monitoring (Park et al., 2023).
Informações legais
produto relacionado
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 2
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
35.6 °F - closed cup
Ponto de fulgor (°C)
2.0 °C - closed cup
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lamentamos, não temos COA para este produto disponíveis online no momento.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica