810227P
Avanti
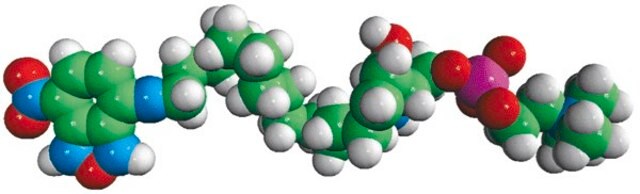
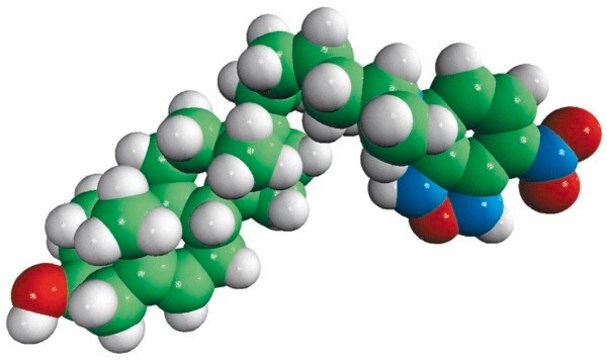
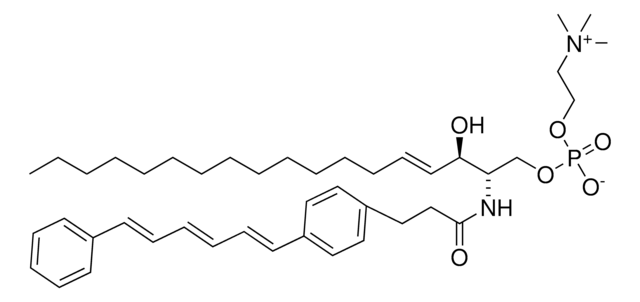
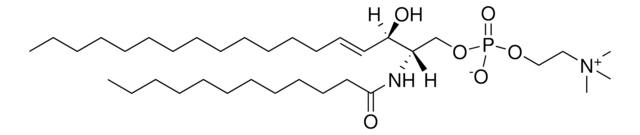
C12-NBD Lactosyl Ceramide
Avanti Research™ - A Croda Brand 810227P, powder
Sinônimo(s):
N-[12-[(7-nitro-2-1,3-benzoxadiazol-4-yl)amino]dodecanoyl]-D-lactosyl-β1-1′-sphingosine
About This Item
Produtos recomendados
Ensaio
>99% (TLC)
Formulário
powder
embalagem
pkg of 1 × 50 μg (810227P-50ug)
fabricante/nome comercial
Avanti Research™ - A Croda Brand 810227P
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
Descrição geral
Ações bioquímicas/fisiológicas
Embalagem
Informações legais
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Certificados de análise (COA)
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica