800725P
Avanti
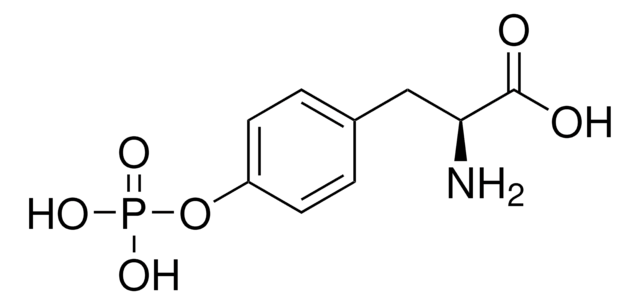
N-P Tyrosine PA
Avanti Research™ - A Croda Brand 800725P, powder
Sinônimo(s):
N-palmitoyl-tyrosine phosphoric acid (ammonium salt)
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C25H48N3O7P
Número CAS:
Peso molecular:
533.64
Número MDL:
Código UNSPSC:
12352211
NACRES:
NA.25
Produtos recomendados
Ensaio
>99% (TLC)
Formulário
powder
embalagem
pkg of 1 × 1 mg (800725P-1mg)
fabricante/nome comercial
Avanti Research™ - A Croda Brand 800725P
tipo de lipídio
phospholipids
cardiolipins
Condições de expedição
dry ice
temperatura de armazenamento
−20°C
Descrição geral
Lysophosphatidic acid (LPA) receptor modulators include N-palmitoyl serine phosphoric acid and N-palmitoyl-tyrosine phosphoric acid. N-palmitoyl serine phosphoric acid and N-palmitoyl-tyrosine phosphoric acid are competitive inhibitors of the LPA receptor in Xenopus oocytes. However, in mammalian cells, N-palmitoyl-tyrosine phosphoric acid may act as an agonist for the LPA receptor. LPA is a lipid mediator that acts similar to growth factors through G-protein coupled plasma membrane receptors. LPA may play a role in platelet aggregation, smooth muscle contraction, vasoactive changes, cytoskeletal reorganization and cell proliferation.
Embalagem
5 mL Amber Glass Screw Cap Vial (800725P-1mg)
Nota de preparo
Product use: N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acid can be used for cell studies. dissolved these lipids in 0.1 mL PBS containing 0.1 mg/mL human serum albumin before adding to cells. In X. laevis studies, these LPA inhibitors were dissolved in DMSO at 1 mM and filtered through a 0.45 mM membrane filter before injection.
Informações legais
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Código de classe de armazenamento
11 - Combustible Solids
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Lot/Batch Number
It looks like we've run into a problem, but you can still download Certificates of Analysis from our Documentos section.
Se precisar de ajuda, entre em contato Atendimento ao cliente
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Hydrolysis of phosphatidylserine-exposing red blood cells by secretory phospholipase A2 generates lysophosphatidic acid and results in vascular dysfunction.
Neidlinger, N.A, et. al.
The Journal of Biological Chemistry, 281, 775-781 (2006)
Inhibitors of lipid phosphatidate receptors: N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acids
Bittman, R, et. al.
Journal of Lipid Research, 37, 391-398 (1996)
Recombinant human G protein-coupled lysophosphatidic acid receptors mediate intracellular calcium mobilization
An, S
Molecular Pharmacology, 54, 881-888 (1998)
Inhibitors of lipid phosphatidate receptors: N-palmitoyl-serine and N-palmitoyl-tyrosine phosphoric acids
Bittman, R
Journal of Lipid Research, 37, 391-398 (1996)
R Bittman et al.
Journal of lipid research, 37(2), 391-398 (1996-02-01)
An improved synthesis of two lipid phosphoric acids, N-palmitoyl-L-serine phosphoric acid (NP-Ser-PA) and N-palmitoyl-L-tyrosine phosphoric acid (NP-Tyr-PA), from the benzyl esters of L-serine and L-tyrosine is described. The sequence of N-acylation, followed by phosphitylation with N, N-diisopropyl dibenzyl phosphoramidite, oxidation
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica