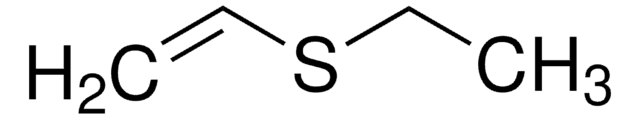G7208
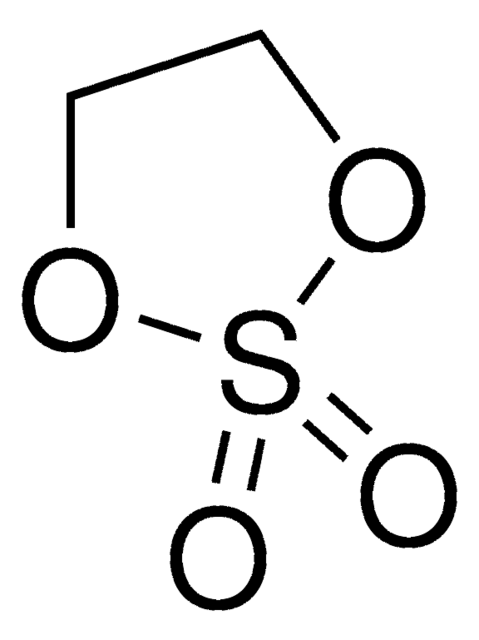
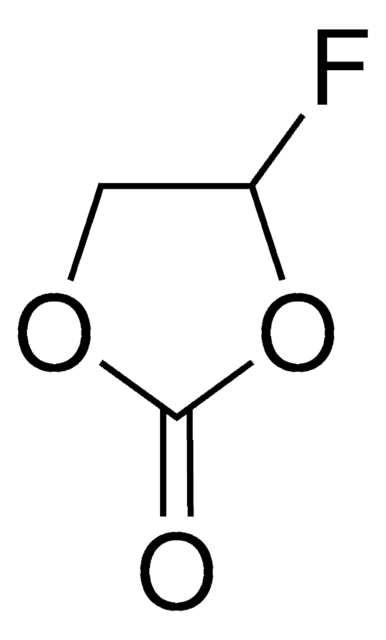
Ethylene sulfite
98%
Sinônimo(s):
1,3,2-Dioxathiolan-2-oxide, Cyclic ethylene sulfite, ES, Glycol sulfite
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C2H4O3S
Número CAS:
Peso molecular:
108.12
Beilstein:
1237109
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
98%
Formulário
liquid
índice de refração
n20/D 1.445 (lit.)
p.e.
159.1 °C (lit.)
densidade
1.426 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
O=S1OCCO1
InChI
1S/C2H4O3S/c3-6-4-1-2-5-6/h1-2H2
chave InChI
WDXYVJKNSMILOQ-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
197.1 °F
Ponto de fulgor (°C)
91.7 °C
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Joseph P O'Shea et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 96, 207-216 (2015-07-29)
Novel formulations that overcome the solubility limitations of poorly water soluble drugs (PWSD) are becoming ever more critical to a drug development process inundated with these compounds. There is a clear need for developing bio-enabling formulation approaches to improve oral
Dharmendra K Yadav et al.
AAPS PharmSciTech, 16(4), 855-864 (2015-01-15)
The objective of this study was to develop novel docetaxel phospholipid nanoparticles (NDPNs) for intravenous administration. Modified solvent diffusion-evaporation method was adopted in the NDPN preparation. Central composite design (CCD) was employed in the optimization of the critical formulation factor
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(6), 1734-1746 (2014-04-18)
The current study determined the extent to which the desorption of lipid-based formulations (LBFs) from a mesoporous magnesium aluminometasilicate (Neusilin®-US2) carrier is governed by drug properties, LBF composition, and LBF-to-adsorbent ratio. A secondary objective was to evaluate the impact of
Hywel D Williams et al.
Journal of pharmaceutical sciences, 103(8), 2441-2455 (2014-07-06)
The Lipid Formulation Classification System Consortium looks to develop standardized in vitro tests and to generate much-needed performance criteria for lipid-based formulations (LBFs). This article highlights the value of performing a second, more stressful digestion test to identify LBFs near
Mette U Anby et al.
Pharmaceutical research, 31(6), 1536-1552 (2014-01-31)
To explore the possibility that age-related changes in physiology may result in differences in drug bioavailability after oral administration of lipid based formulations of danazol. Danazol absorption from lipid formulations with increasing drug load was examined in younger (9 months)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica