C100005
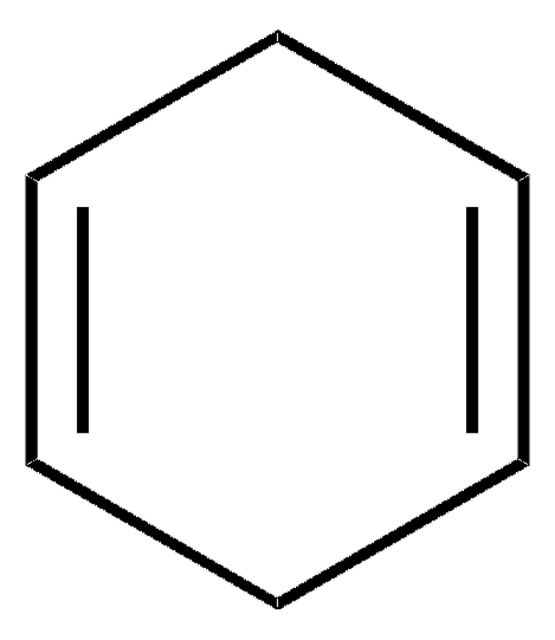
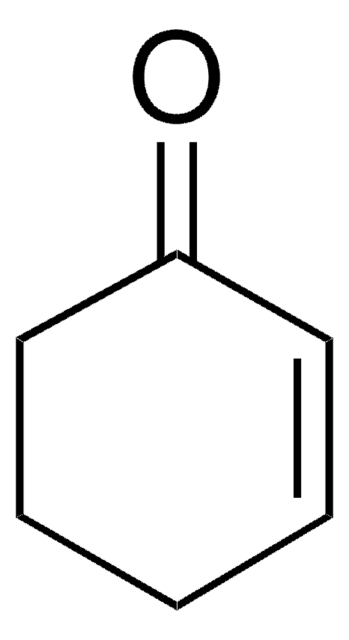
1,3-Cyclohexadiene
contains 0.05% BHT as inhibitor, 97%
Sinônimo(s):
1,2-Dihydrobenzene
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C6H8
Número CAS:
Peso molecular:
80.13
Beilstein:
506024
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
97%
Formulário
liquid
contém
0.05% BHT as inhibitor
índice de refração
n20/D 1.474 (lit.)
p.e.
80 °C (lit.)
densidade
0.841 g/mL at 25 °C (lit.)
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
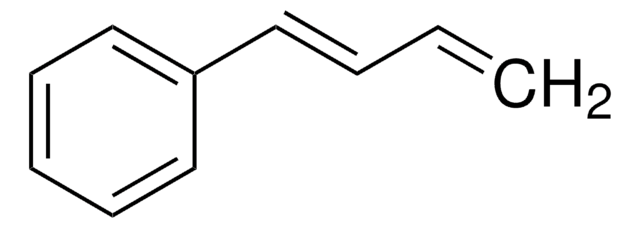
C1CC=CC=C1
InChI
1S/C6H8/c1-2-4-6-5-3-1/h1-4H,5-6H2
chave InChI
MGNZXYYWBUKAII-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Aplicação
1,3-Cyclohexadiene can undergo:
- C-C coupling with aromatic alcohols via iridium-catalyzed hydrogen auto-transfer and with aldehydes via transfer hydrogenation mediated by isopropanol to form carbonyl addition products.
- Living anionic polymerization with n-BuLi/TMEDA system to form polycyclohexadiene.
- Platinum-catalyzed silaboration to form (1R,4S)-1-(dimethylphenylsilyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-2-cyclohexene.
- Aerobic palladium-catalyzed 1,4-diacetoxylation in the presence of cobalt tetra(hydroquinone)porphyrin as an electron transfer reagent.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Flam. Liq. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
3 - Flammable liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
66.0 °F
Ponto de fulgor (°C)
18.9 °C
Equipamento de proteção individual
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Sarah J Ryan et al.
Journal of the American Chemical Society, 133(13), 4694-4697 (2011-03-12)
Herein we report the first all-carbon N-heterocyclic carbene-catalyzed (4 + 2) cycloaddition. The reaction proceeds with α,β-unsaturated acid fluorides and silyl dienol ethers and produces 1,3-cyclohexadienes with complete diastereocontrol (dr >20:1) while demonstrating a new type of reaction cascade exploiting
Contact formation dynamics: Mapping chemical bond formation between a molecule and a metallic probe.
Borislav Naydenov et al.
Nano letters, 6(8), 1752-1756 (2006-08-10)
We present a study that maps out chemical bond formation between a Pt-inked probe and a single 1,3-cyclohexadiene (1,3-CHD) molecule on Si(100). By separating the mechanical and electronic contributions to the current during the approach to contact, we show that
Enantioselective Platinum?Catalyzed Silicon?Boron Addition to 1, 3?Cyclohexadiene.
Gerdin M and Moberg C
Advanced Synthesis & Catalysis, 347(6), 749-753 (2005)
Marija Kotur et al.
The Journal of chemical physics, 130(13), 134311-134311 (2009-04-10)
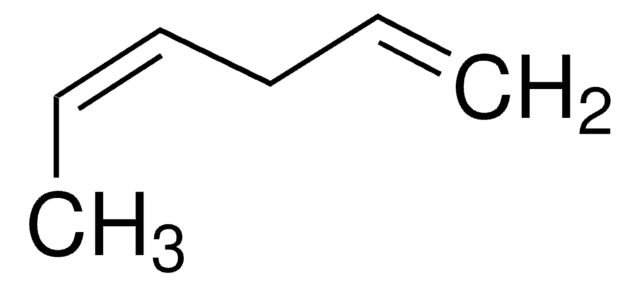
We demonstrate the use of shaped ultrafast laser pulses in the deep ultraviolet to control the ring opening isomerization of 1,3-cyclohexadiene to form 1,3,5-hexatriene. The experiments are performed with a gas phase sample and the isomerization yield is probed with
Chemo-, regio-, and stereoselective cobalt-mediated [2+2+2] cycloaddition of alkynyl boronates to alkenes: 1,3- and 1,4-diboryl-1,3-cyclohexadienes.
Vincent Gandon et al.
Angewandte Chemie (International ed. in English), 44(43), 7114-7118 (2005-10-12)
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica