About This Item
Fórmula empírica (Notação de Hill):
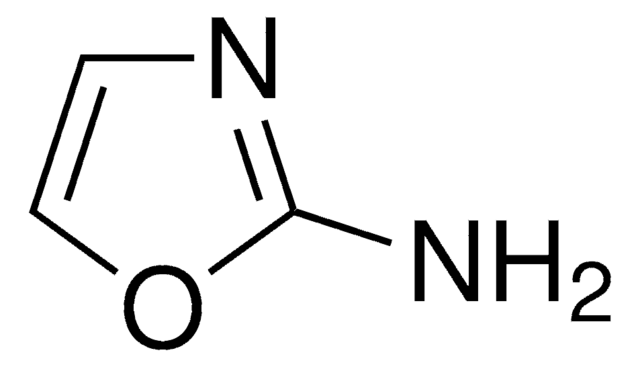
C3H4N2O
Número CAS:
Peso molecular:
84.08
Número MDL:
Código UNSPSC:
12352100
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Nível de qualidade
Ensaio
95%
Formulário
liquid
índice de refração
n20/D 1.511 (lit.)
p.e.
226-228 °C (lit.)
densidade
1.138 g/mL at 25 °C (lit.)
cadeia de caracteres SMILES
Nc1ccon1
InChI
1S/C3H4N2O/c4-3-1-2-6-5-3/h1-2H,(H2,4,5)
chave InChI
RHFWLPWDOYJEAL-UHFFFAOYSA-N
Descrição geral
3-Aminoisoxazole (isoxazol-3-amine) is a 3-substituted isoxazole derivative. It is a structural isomer of 5-aminoisoxazole.
Aplicação
3-Aminoisoxazole (isoxazol-3-amine) may be used in the following studies:
- As a reagent in the synthesis of N-(4-(N-isoxazol-3-ylsulfamoyl)phenyl)acetamide.
- As a starting material in the synthesis of N-(isoxazol-3-yl)-N′-(carbomethoxy)thiourea.
- As a starting material in the synthesis of (Z)-2-(5-amino-1,2,4-thiadiazol-3-yl)-2-methoxy-iminoacetic acid, a side-chain of the fourth generation of cephem antibiotics.
Palavra indicadora
Warning
Frases de perigo
Declarações de precaução
Classificações de perigo
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órgãos-alvo
Respiratory system
Código de classe de armazenamento
10 - Combustible liquids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
235.4 °F
Ponto de fulgor (°C)
113 °C
Equipamento de proteção individual
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Synthesis of 3-and 5-amino-5-(3)-(pyrrol-2-yl) isoxazoles.
Lyubov'N S, et al.
Tetrahedron, 61(20), 4841-4849 (2005)
Martin J. Walsh et al.
Probe Reports from the NIH Molecular Libraries Program, 2011 Oct 18 (Updated 2013 Feb 25) (2013-09-13)
The protist
Tomasz Glinka et al.
Bioorganic & medicinal chemistry, 11(4), 591-600 (2003-01-23)
SAR studies in a series of related 3-(heteroarylthio)cephems determined that a relatively high chemical reactivity of the beta-lactam ring, modulated by electronic effects of substituents at C-3 and C-7, is necessary to achieve high in vitro activity against methicillin-resistant Staphylococcus
Kuniaki Tatsuta
Proceedings of the Japan Academy. Series B, Physical and biological sciences, 84(4), 87-106 (2008-10-23)
The first total synthesis and development of a variety of bioactive natural products have been accomplished by using carbohydrates as a chiral source. In addition, practically useful intermediates have been created, analogs of natural products have been prepared, their structure-activity
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica