90827
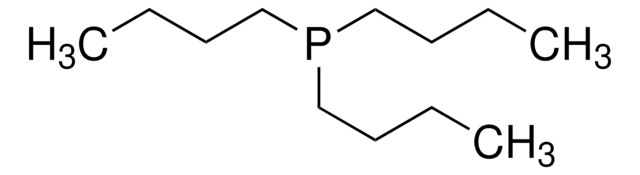
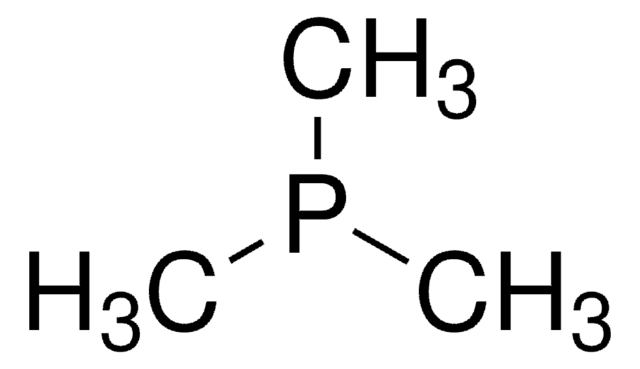
Tributylphosphine
≥93.5% (Tri-N-butylphosphine, GC)
Sinônimo(s):
P(n-Bu)3, TBP
About This Item
Produtos recomendados
densidade de vapor
9 (vs air)
Ensaio
≥93.5% (Tri-N-butylphosphine, GC)
≥97% (Tri-N-butylphospine + isomers)
Formulário
liquid
temperatura de autoignição
392 °F
adequação da reação
reaction type: Acetylations
reagent type: ligand
índice de refração
n20/D 1.462 (lit.)
n20/D 1.463
p.e.
150 °C/50 mmHg (lit.)
densidade
0.81 g/mL at 25 °C (lit.)
grupo funcional
phosphine
cadeia de caracteres SMILES
CCCCP(CCCC)CCCC
InChI
1S/C12H27P/c1-4-7-10-13(11-8-5-2)12-9-6-3/h4-12H2,1-3H3
chave InChI
TUQOTMZNTHZOKS-UHFFFAOYSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Descrição geral
Aplicação
It may be used in the following processes:
- As reducing agent for alkyl disulfides and aromatic disulfides.
- As catalyst for the synthesis of 2-substituted 1,3-benzoselenazoles.
- As promoter for the ring opening of epoxides and aziridines with nucleophiles.
- As a reagent in the preparation of 6-substituted penicillanate esters by reduction of 6-bromo-6-substituted penicillanate esters in high diastereoselectivity.
- As a catalyst in the acylation reaction of alcohols.
- As a catalyst to prepare rotaxanes by the acylation of corresponding pseudorotaxanes using 3,5-dimethylbenzoic anhydride.
- As a catalyst to prepare vinyl thioethers by the Michael addition of ethanethiol to various alkynyl ketones.
- As a promoter in the conjugate addition of non-nucleophilic N-containing compounds with Michael acceptors.
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Pyr. Liq. 1 - Skin Corr. 1A
Código de classe de armazenamento
4.2 - Pyrophoric and self-heating hazardous materials
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
242.6 °F - closed cup
Ponto de fulgor (°C)
117 °C - closed cup
Equipamento de proteção individual
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica