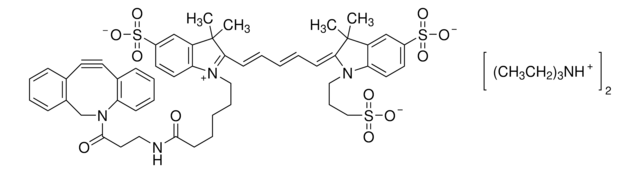
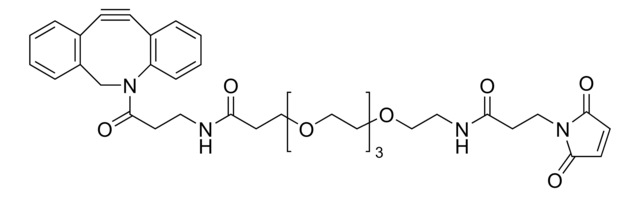
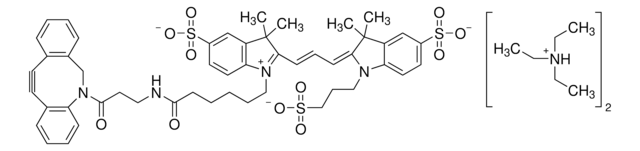
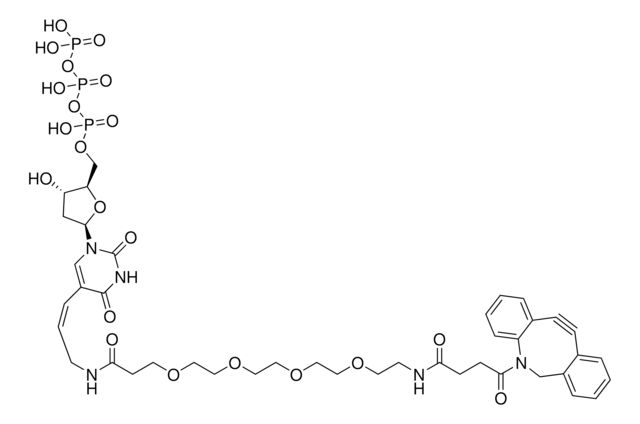
761540
Dibenzocyclooctyne-amine
for Copper-free Click Chemistry
Sinônimo(s):
DBCO-NH2, DBCO-amine
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥94.5 %
Formulário
solid
adequação da reação
reaction type: click chemistry
reagent type: linker
pf
86-96 °C
grupo funcional
amine
temperatura de armazenamento
−20°C
cadeia de caracteres SMILES
NCCC(N1CC2=C(C=CC=C2)C#CC3=C1C=CC=C3)=O
InChI
1S/C18H16N2O/c19-12-11-18(21)20-13-16-7-2-1-5-14(16)9-10-15-6-3-4-8-17(15)20/h1-8H,11-13,19H2
chave InChI
OCCYFTDHSHTFER-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
Aplicação
- Linker to link azide-containing functional groups. It facilitates the formation of linkages through Strain-promoted azide-alkyne cycloaddition reactions (SPAAC)
- Reagent in the synthesis of N-heterocyclic carbene metal thiolates. It functionalizes the metal complexes and increases their reactivity in the strain-promoted alkyne–azide cycloaddition (SPAAC) reactions
- Amine functionalized cyclooctyne derivative. Cyclooctynes are useful in strain-promoted copper-free azide-alkyne cycloaddition reactions. This dibenzocyclooctyne will react with azide functionalized compounds or biomolecules without the need for a Cu(I) catalyst to result in a stable triazole linkage.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Os clientes também visualizaram
Artigos
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 761540-100MG | 4061832918426 |
| 761540-10MG | 4061833230619 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/171/632/0556139a-2db5-4678-a6ec-a26a693fd574/640/0556139a-2db5-4678-a6ec-a26a693fd574.png)
![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)