726508
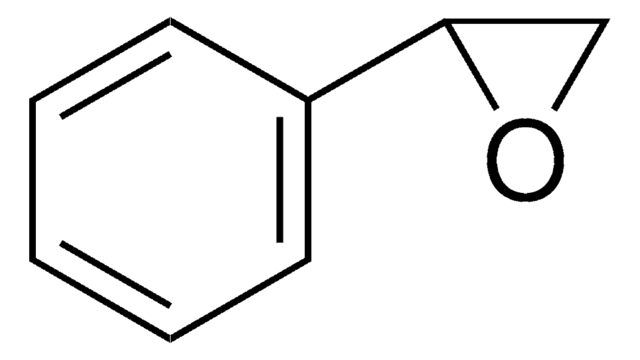
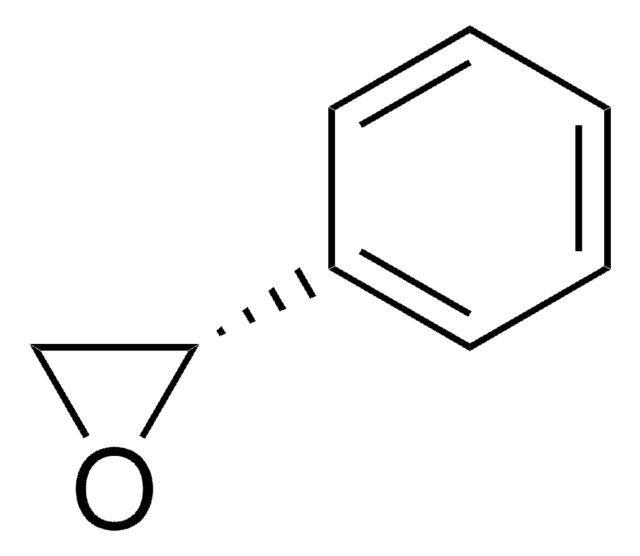
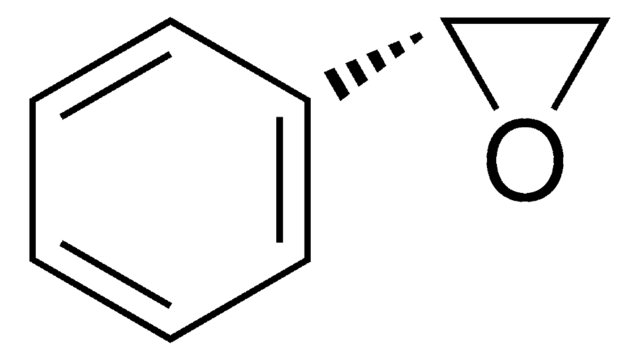
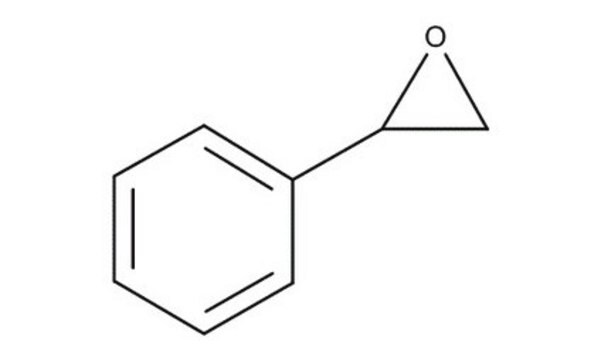
(R)-(+)-Styrene oxide
ChiPros®, produced by BASF, ≥98%
Sinônimo(s):
(R)-(+)-Phenyloxirane, (R)-Phenylethylene oxide
About This Item
Produtos recomendados
Nível de qualidade
Ensaio
≥98%
≥98.0% (GC)
Formulário
liquid
pureza óptica
enantiomeric excess: ≥98.0%
Lim. expl.
~22 %
índice de refração
n20/D 1.534 (lit.)
p.e.
89-90 °C/23 mmHg (lit.)
densidade
1.051 g/mL at 25 °C (lit.)
grupo funcional
ether
phenyl
cadeia de caracteres SMILES
C1O[C@@H]1c2ccccc2
InChI
1S/C8H8O/c1-2-4-7(5-3-1)8-6-9-8/h1-5,8H,6H2/t8-/m0/s1
chave InChI
AWMVMTVKBNGEAK-QMMMGPOBSA-N
Procurando produtos similares? Visita Guia de comparação de produtos
Categorias relacionadas
Informações legais
Palavra indicadora
Danger
Frases de perigo
Declarações de precaução
Classificações de perigo
Acute Tox. 4 Dermal - Carc. 1B - Eye Irrit. 2
Código de classe de armazenamento
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de risco de água (WGK)
WGK 3
Ponto de fulgor (°F)
176.0 °F - closed cup
Ponto de fulgor (°C)
80 °C - closed cup
Escolha uma das versões mais recentes:
Certificados de análise (COA)
Não está vendo a versão correta?
Se precisar de uma versão específica, você pode procurar um certificado específico pelo número do lote ou da remessa.
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Artigos
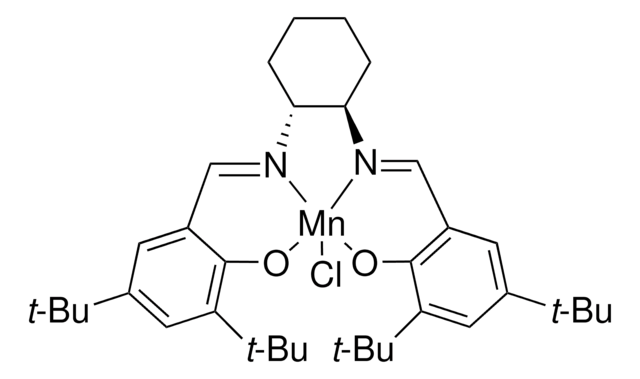
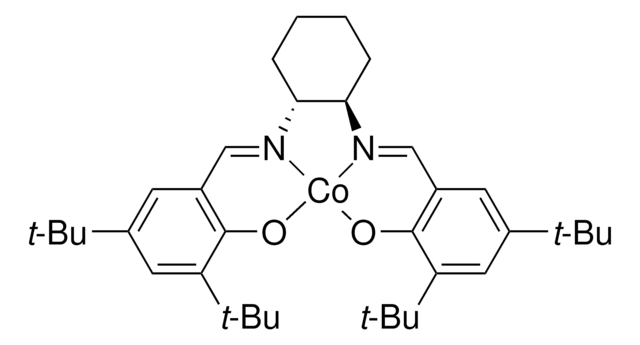
There are several alternative routes towards chiral aryl-substituted epoxides, among them Jacobsen’s asymmetric epoxidation1 or his hydrolytic kinetic resolution2 method, Sharpless’s asymmetric epoxidation3 using catalytic titan(IV)- isopropylate/diethyl tartrate complexes and tert-butylhydroperoxide
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 726508-100G | 4061832719795 |
| 726508-25G | 4061832719801 |
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica