704415
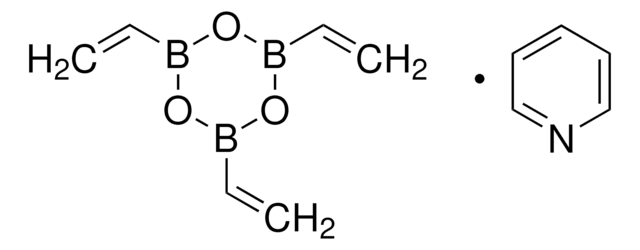
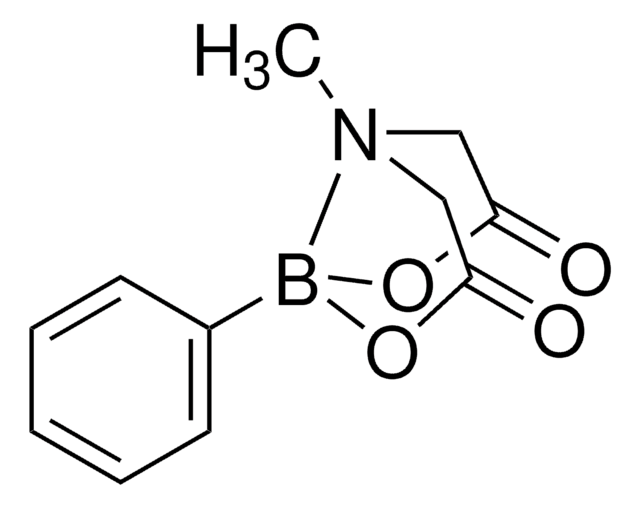
Vinylboronic acid MIDA ester
97%
Sinônimo(s):
6-Methyl-2-vinyl-1,3,6,2-dioxazaborocane-4,8-dione
Faça loginpara ver os preços organizacionais e de contrato
About This Item
Fórmula empírica (Notação de Hill):
C7H10BNO4
Número CAS:
Peso molecular:
182.97
Número MDL:
Código UNSPSC:
12352103
ID de substância PubChem:
NACRES:
NA.22
Produtos recomendados
Ensaio
97%
Formulário
powder
pf
152-156 °C
temperatura de armazenamento
2-8°C
cadeia de caracteres SMILES
CN1CC(=O)OB(OC(=O)C1)C=C
InChI
1S/C7H10BNO4/c1-3-8-12-6(10)4-9(2)5-7(11)13-8/h3H,1,4-5H2,2H3
chave InChI
MGRQGYAVASCCAK-UHFFFAOYSA-N
Categorias relacionadas
Descrição geral
Vinylboronic acid MIDA ester, like other MIDA boronates, possesses the capacity for controlled, in situ slow-release of boronic acids under aqueous basic conditions allowing the cross-coupling of classically challenging substrates.
Aplicação
MIDA boronates as stable boronic acid surrogates for classically challenging cross-couplings
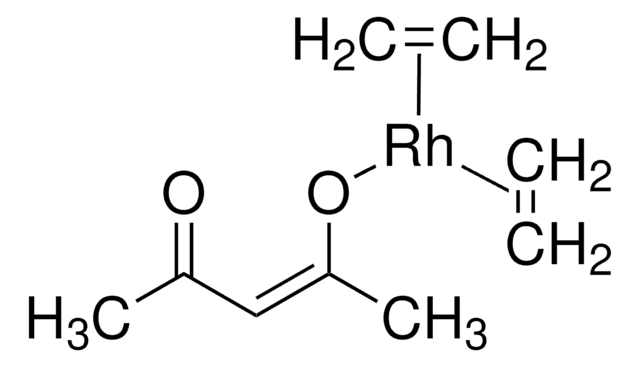
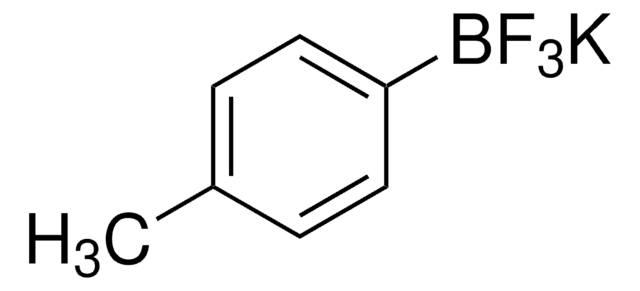
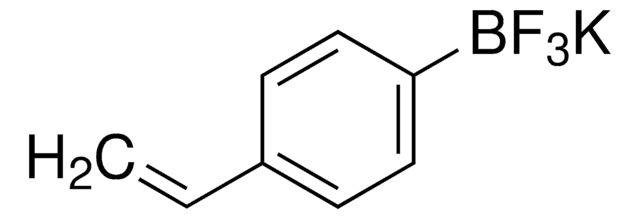
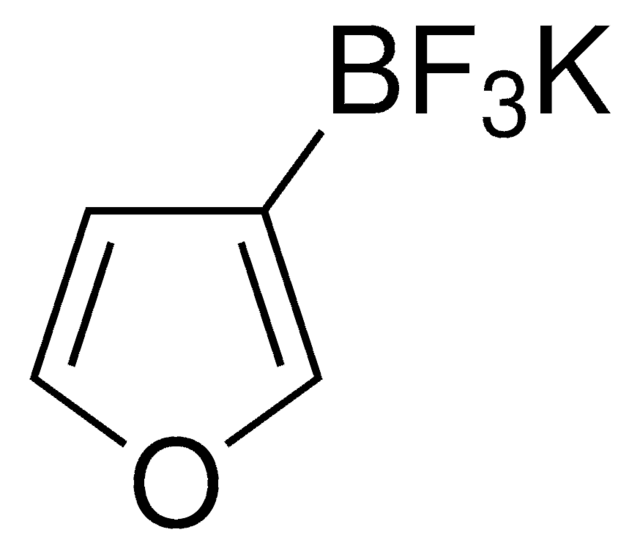
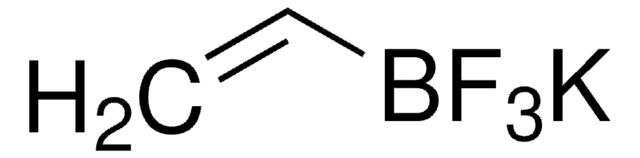
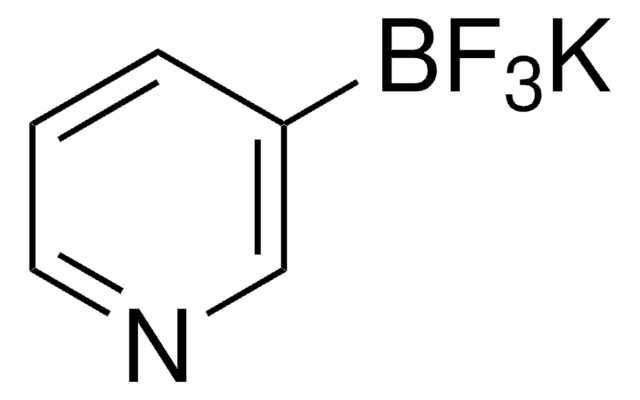
Suzuki Cross-Coupling with MIDA Boronates
Suzuki Cross-Coupling with MIDA Boronates
- Vinylboronic acid MIDA ester is an air and chromatographically stable boronic acid surrogate for Suzuki-Miyaura cross-coupling. It can also be used in Heck and oxidative Heck reactions as well as in olefin metathesis to provide the cross-coupled product.
- It is compatible with a wide range of common synthetic reagents that allows functionalization to synthesize structurally complex boronic acid surrogates.
- It undergoes cyclopropanation and epoxidation to yield corresponding MIDA cyclopropane and oxirane, respectively.
- It can be used as one of the major reagents for the scalable synthesis of potent cytotoxin, Leiodermatolide and for the total synthesis of (−)-Blepharocalyxin D.
Código de classe de armazenamento
11 - Combustible Solids
Classe de risco de água (WGK)
WGK 2
Ponto de fulgor (°F)
Not applicable
Ponto de fulgor (°C)
Not applicable
Equipamento de proteção individual
Eyeshields, Gloves, type N95 (US)
Escolha uma das versões mais recentes:
Já possui este produto?
Encontre a documentação dos produtos que você adquiriu recentemente na biblioteca de documentos.
Vinyl MIDA boronate: a readily accessible and highly versatile building block for small molecule synthesis.
Uno BE, et al.
Tetrahedron, 65(16), 3130-3138 (2009)
Synthesis, molecular editing, and biological assessment of the potent cytotoxin leiodermatolide.
Mailhol D, et al.
Journal of the American Chemical Society, 136(44), 15719-15729 (2014)
Synthesis of trans-2-(Trifluoromethyl) cyclopropanes via Suzuki reactions with an N-methyliminodiacetic acid boronate.
Duncton MA and Singh R.
Organic Letters, 15(17), 4284-4287 (2013)
Total Synthesis of (−)-Blepharocalyxin D and Analogues.
Cons BD, et al.
Organic Letters, 15(8), 2046-2049 (2013)
A general solution for unstable boronic acids: slow-release cross-coupling from air-stable MIDA boronates.
Knapp DM, et al.
Journal of the American Chemical Society, 131(20), 6961-6963 (2009)
Artigos
An article regarding MIDA-protected Boronate Esters.
Nossa equipe de cientistas tem experiência em todas as áreas de pesquisa, incluindo Life Sciences, ciência de materiais, síntese química, cromatografia, química analítica e muitas outras.
Entre em contato com a assistência técnica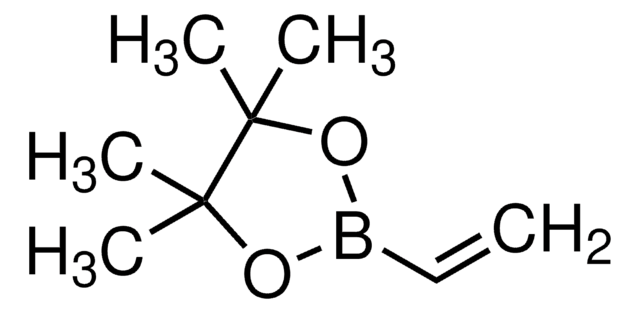
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)